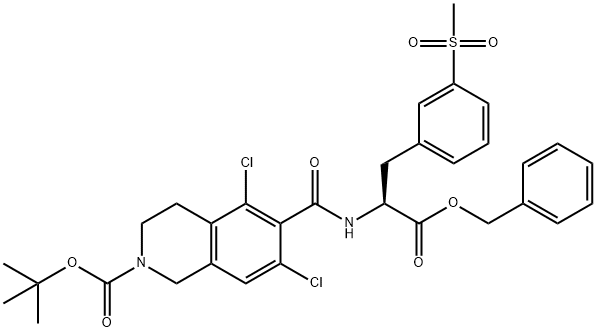
2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester
- CAS Number:1194550-61-2
- Molecular formula:C32H34Cl2N2O7S
- Molecular Weight:661.59
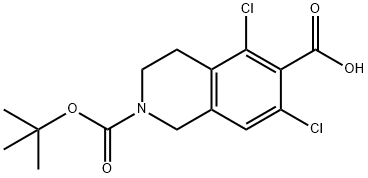
851784-82-2
172 suppliers
$11.00/250mg
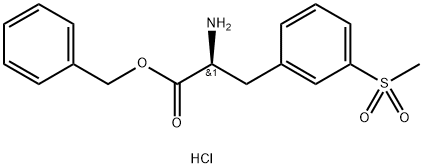
1194550-59-8
130 suppliers
$43.00/1g
![2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1194550-61-2.gif)
1194550-61-2
28 suppliers
inquiry
Yield:1194550-61-2 170 g
Reaction Conditions:
with 1-hydroxy-7-aza-benzotriazole;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in N,N-dimethyl-formamide at 20;Reagent/catalyst;Solvent;
Steps:
1 Example 1: Preparation of compound of formula V
To a solution of 2-(tert-butoxycarbonyl)-5,7-dichloro-l,2,3,4-tetrahydroisoquinoline-6- carboxylic acid (compound VII, lOOgm) and Benzyl (,S)-2-amino-3-[3- (methylsulfonyl)phenyl]propionate hydrochloride (compound VI, 112.17gm) in DMF was added EDC.HC1 (83gm), HOAt (58.96gm) followed by triethyl amine (87.6gm) under stirring at room temperature. After completion of reaction, the reaction mass was added slowly to water, stirred for 2-3 hours and the precipitated solid was filtered. The solid obtained was washed with water. The wet cake was stirred in 5% aq. potassium carbonate and filtered. The solid was washed with water and dried under vacuum at 40- 45°C for 12 hours to afford the title compound (0324) Yield: 170 gm, HPLC Purity: 95.68 %.
References:
WO2019/53607,2019,A1 Location in patent:Paragraph 0207
10203-08-4
294 suppliers
$8.00/5g
![2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1194550-61-2.gif)
1194550-61-2
28 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1194550-61-2.gif)
1194550-61-2
28 suppliers
inquiry
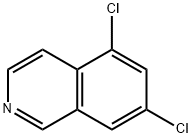
73075-58-8
18 suppliers
inquiry
![2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1194550-61-2.gif)
1194550-61-2
28 suppliers
inquiry
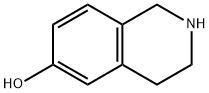
14446-24-3
187 suppliers
$23.00/250mg
![2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1194550-61-2.gif)
1194550-61-2
28 suppliers
inquiry