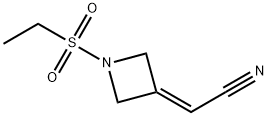
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile synthesis
- Product Name:2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile
- CAS Number:1187595-85-2
- Molecular formula:C7H10N2O2S
- Molecular Weight:186.23
1314910-43-4
82 suppliers
inquiry
594-44-5
253 suppliers
$21.00/25g

1187595-85-2
266 suppliers
inquiry
Yield:1187595-85-2 98.59%
Reaction Conditions:
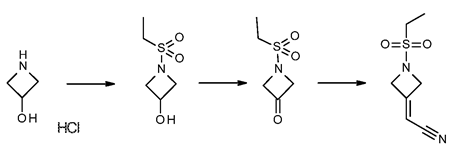
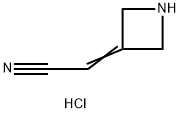
Stage #1: 2-(azetidin-3-ylidene)acetonitrile hydrochloridewith N-ethyl-N,N-diisopropylamine in acetonitrile at 0 - 10; for 0.166667 h;
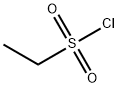
Stage #2: Ethanesulfonyl chloride in acetonitrile at 0 - 25; for 16 h;
Steps:
2 Preparation of 2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile (Formula II)
N,N-Diisopropylethylamine (4.5 mL) was added into a reaction vessel containing acetonitrile (50 mL) and 3-(cyanomethylene)azetidine hydrochloride (1.5 g; Formula VII) at about 0°C to about 10°C. The reaction mixture was stirred for about 10 minutes. Ethanesulfonyl chloride (2.22 g) was added into the reaction mixture at about 0°C to about 5°C over about 5 minutes. The temperature of the reaction mixture was raised to about 20°C to about 25 °C, and then the reaction mixture was stirred for about 16 hours. On completion of the reaction, acetonitrile was recovered from the reaction mixture under reduced pressure at about 40°C to about 45°C to obtain an oily residue. Dichloromethane (50 mL) was added into the residue. The contents were washed with a saturated sodium chloride solution (30 mL), followed by complete recovery of dichloromethane under reduced pressure at about 40°C to obtain 2-(l-(ethylsulfonyl)azetidin-3- ylidene)acetonitrile . Yield: 98.59%
References:
WO2016/125080,2016,A2 Location in patent:Page/Page column 11
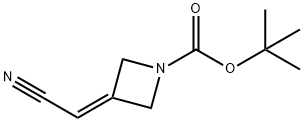
1153949-11-1
189 suppliers
$14.00/1g

1187595-85-2
266 suppliers
inquiry