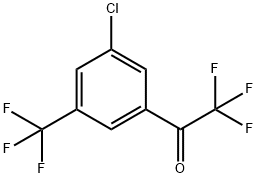
1-[3-Chloro-5-trifluoromethylphenyl]-2,2,2-trifluoroethanone synthesis
- Product Name:1-[3-Chloro-5-trifluoromethylphenyl]-2,2,2-trifluoroethanone
- CAS Number:1125812-58-9
- Molecular formula:C9H3ClF6O
- Molecular Weight:276.56

Cool the solution containing compound III (74.4g, 0.255mol containing compound III) to -100, and add 120g of sulfuric acid with a mass fraction of 92.5% to the above system while keeping the temperature below 0. After acidification, continue to react and keep for 1.5h, and then add 126.7g of 33% sodium nitrite aqueous solution (containing diazotizing reagent sodium nitrite 0.612mol) into the system uniformly within 2h, and keep the temperature at 0 The following reaction is carried out for 2 hours, and the system is heated to room temperature after full reaction, 111.1 g of 50% hypophosphorous acid and 0.67 g of cuprous oxide are added, and the reaction is stirred for 2 hours while keeping temperature. After the reaction is over, stand to separate the layers, and wash the organic layer with a 5% sodium bicarbonate aqueous solution, dry with anhydrous magnesium sulfate, and rectify to obtain a clear and transparent oily liquid 1-[3-chloro-5-(trifluoromethyl) (Yl)phenyl]-2,2,2-trifluoroethanone 66.9, content 99.5%, yield 87.2%.
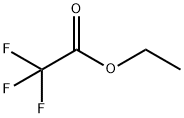
383-63-1
484 suppliers
$10.00/25 g
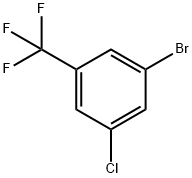
928783-85-1
147 suppliers
$5.00/1g
![1-[3-Chloro-5-trifluoromethylphenyl]-2,2,2-trifluoroethanone](/CAS2/GIF/1125812-58-9.gif)
1125812-58-9
140 suppliers
$21.00/100mg
Yield:1125812-58-9 78%
Reaction Conditions:
Stage #1:1-bromo-3-chloro-5-(trifluoromethyl)benzene with magnesium in diethyl ether at 0; for 0.5 h;Inert atmosphere;
Stage #2:ethyl trifluoroacetate, in diethyl ether at -80; for 0.5 h;Inert atmosphere;
Stage #3: with hydrogenchloride in diethyl ether;water at -10;
Steps:
4.1
In a 500 ml multineck flask with low-temperature thermometer, dropping funnel and argon balloon, 5.5 g (0.228 mol, 1.3 eq) of magnesium turnings (activated with dibromoethane and washed with diethyl ether) were blanketed with 120 ml of diethyl ether. At 0° C., 45.5 g of 3-bromo-5-chlorobenzotrifluoride (0.175 mol, 1 eq) in 120 ml of diethyl ether were slowly added dropwise. The reaction started up of its own accord after a few minutes, with a colour change (red-brown) and heating; the temperature was kept at about 0° C. during the addition. On completion of addition of the bromide, the mixture was stirred for a further 30 min.A separate 2 l 3-neck flask with low-temperature thermometer, dropping funnel and argon balloon was initially charged with 32.4 g of ethyl trifluoroacetate (0.228 mol, 1.3 eq) in 250 ml of diethyl ether, and cooled to -80° C. At this temperature, the Grignard reagent (cooled to -10° C.) was slowly added dropwise. On completion of addition, the reaction mixture was stirred at -80° C. for a further 30 min. Thereafter, the reaction mixture was warmed to -10° C. and acidified with 10% hydrochloric acid. The resulting mixture was admixed with saturated NaCl solution, the phases were separated and the aqueous phase was washed with 200-300 ml of diethyl ether. The combined ethereal phases were dried over magnesium sulphate and the solvent was subsequently removed on a rotary evaporator.The resulting crude product was purified by vacuum distillation (71° C. at 18 mbar). 37.7 g (78% of theory) of 1-[3-chloro-5-(trifluoromethyl)phenyl]-2,2,2-trifluoroethanone were obtained as a colourless oil. This was converted further without further purification.
References:
Bayer CropScience AG US2011/105532, 2011, A1 Location in patent:Page/Page column 40
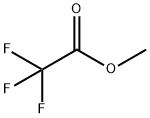
431-47-0
285 suppliers
$10.00/5g
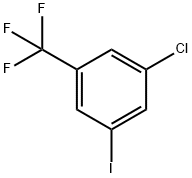
1189352-83-7
67 suppliers
$15.00/250mg
![1-[3-Chloro-5-trifluoromethylphenyl]-2,2,2-trifluoroethanone](/CAS2/GIF/1125812-58-9.gif)
1125812-58-9
140 suppliers
$21.00/100mg
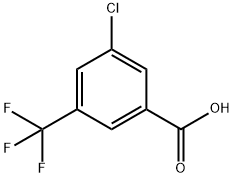
53985-49-2
94 suppliers
$14.00/1g
![1-[3-Chloro-5-trifluoromethylphenyl]-2,2,2-trifluoroethanone](/CAS2/GIF/1125812-58-9.gif)
1125812-58-9
140 suppliers
$21.00/100mg
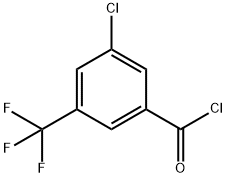
886496-83-9
32 suppliers
$60.00/500 mg
![1-[3-Chloro-5-trifluoromethylphenyl]-2,2,2-trifluoroethanone](/CAS2/GIF/1125812-58-9.gif)
1125812-58-9
140 suppliers
$21.00/100mg
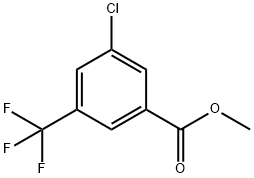
1214361-12-2
20 suppliers
inquiry
![1-[3-Chloro-5-trifluoromethylphenyl]-2,2,2-trifluoroethanone](/CAS2/GIF/1125812-58-9.gif)
1125812-58-9
140 suppliers
$21.00/100mg
