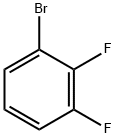
1-Bromo-2,3-difluorobenzene synthesis
- Product Name:1-Bromo-2,3-difluorobenzene
- CAS Number:38573-88-5
- Molecular formula:C6H3BrF2
- Molecular Weight:192.99
 Fig The synthetic method of 1-Bromo-2,3-difluorobenzene
Fig The synthetic method of 1-Bromo-2,3-difluorobenzene
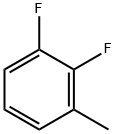
3828-49-7
258 suppliers
$6.00/1g

38573-88-5
377 suppliers
$6.00/1g
Yield:38573-88-5 91%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in dichloromethane at 0 - 40;Large scale;
Steps:
Referring to Figure 1-2, a preparation method of baloxavir intermediate includes adding 6L of dichloromethane and 1kg of 2,3-difluorotoluene into a reaction flask.Add 10g AIBN, add 1.46kg N-bromosuccinimide in batches with a temperature decrease of 0-10°C, increase the reaction temperature by 40°C after the addition, and control the tracking. After the reaction is completed, it is cooled to room temperature, filtered, and the filtrate is washed with water and left to stand for liquid separation.The organic phase was concentrated to obtain 1.47 kg of Intermediate 1, with a yield of 91%.
References:
CN112358464,2021,A Location in patent:Paragraph 0019
356-77-4
2 suppliers
inquiry

38573-88-5
377 suppliers
$6.00/1g
2646-83-5
0 suppliers
inquiry

38573-88-5
377 suppliers
$6.00/1g