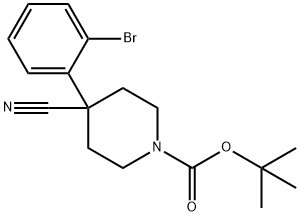
1-BOC-4-CYANO-4-(2-BROMOPHENYL)-PIPERIDINE synthesis
- Product Name:1-BOC-4-CYANO-4-(2-BROMOPHENYL)-PIPERIDINE
- CAS Number:920023-51-4
- Molecular formula:C17H21BrN2O2
- Molecular Weight:365.26
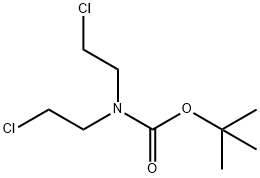
118753-70-1
175 suppliers
$6.00/1g

920023-51-4
20 suppliers
inquiry
Yield:920023-51-4 80%
Reaction Conditions:
Stage #1: (3-bromophenyl)acetonitrilewith sodium hydroxide;tetra(n-butyl)ammonium hydrogensulfate in tetrahydrofuran;water; for 0.166667 h;Heating / reflux;
Stage #2: N-(tert-butyloxycarbonyl)bis(2-chloroethyl)amine in tetrahydrofuran;water at 20; for 4 h;Heating / reflux;
Steps:
282.b
A mixture of 4.50 g (23.0 mmol) 3-bromophenylacetonitrile and 0.78 g (2.3 mmol) tetrabutylammonium hydrogensulfate in 27 ml tetrahydrofuran and 45 ml of a 50% aqueous sodium hydroxide solution was heated at reflux for 10 min. Thereafter 6.11 g (25.3 mmol) bis-(2-chloro-ethyl)-carbamic acid tert-butyl ester were added at room temperature. The reaction mixture was heated at reflux for 4 h. Cooling to room temperature was followed by dilution with 60 ml water and extraction with tert-butyl methyl ether (3*100 ml). The combined organic layers were washed with brine (1*100 ml), dried over sodium sulfate and concentrated in vacuo. The residual crude product was purified by flash chromatography (n-heptane/ethyl acetate) to give the title compound (6.72 g, 80%) as a pale yellow oil. MS m/e (%): 265, 267 (M-BOC+H+, 82, 100).
References:
US2007/27173,2007,A1 Location in patent:Page/Page column 115-116
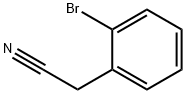
19472-74-3
247 suppliers
$5.00/5g

118753-70-1
175 suppliers
$6.00/1g

920023-51-4
20 suppliers
inquiry

24424-99-5
833 suppliers
$13.50/25G

920023-51-4
20 suppliers
inquiry