1,3,5-TRIACRYLOYLHEXAHYDRO-S-TRIAZINE synthesis
- Product Name:1,3,5-TRIACRYLOYLHEXAHYDRO-S-TRIAZINE
- CAS Number:35216-08-1
- Molecular formula:C12H10O2
- Molecular Weight:186.21
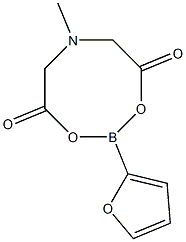
1104637-62-8
10 suppliers
$659.86/1G
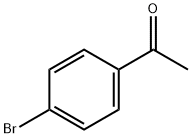
99-90-1
474 suppliers
$6.00/25g

35216-08-1
24 suppliers
inquiry
Yield:35216-08-1 98%
Reaction Conditions:
with potassium phosphate monohydrate;dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane;palladium diacetate in tetrahydrofuran;water at 60; for 24 h;
Steps:
16
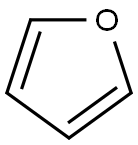
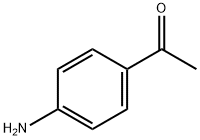
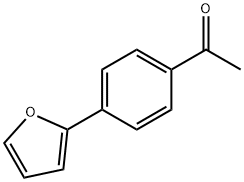
The general method for in situ slow-release of MIDA boronates and cross- coupling with aryl bromides and aryl chlorides was as follows. In a glove box, to a first vial equipped with a stir bar was added 2-dicyclohexylphosphino-2',6'-dimethoxy-1 ,1 '- biphenyl (SPhos), followed by a 0.02 M solution of Pd(OAc)2 in THF in a volume sufficient to afford a 0.04 M solution with respect to the phosphine ligand. The first vial was sealed with a PTFE-lined screwcap and stirred at 23 0C for 30 minutes during which time the orange solution became yellow. Under ambient atmosphere, to a second vial (20 mL) equipped with a stir bar was added the organohalide (1.0 mmol), solid base (7.5 mmol), and the MIDA boronate (1.5 mmol). The second vial was brought into a glove box, and to it was added THF (9.0 mL) and the catalyst stock solution (1 .0 mL) from the first vial. The second vial was sealed with a PTFE-lined septum-screwcap and was removed from the glove box, and to the vial was added degassed H2O (2 mL, sparged with Ar for 30 min.). The resulting biphasic mixture was maintained, with vigorous stirring, at 60 0C for 24 h. The reaction mixture was allowed to cool to 23 0C and then was transferred to a 60 mL separatory funnel. The mixture was diluted with aq. 1 M NaOH (10 mL) and EbO (10 mL), shaken, and then the phases were separated. The aqueous phase was extracted with Et2θ (3 x 10 mL). The combined organic phases were washed with brine (10 mL), were dried over MgSCU and were then filtered. The filtrate was concentrated in vacuo and the resulting residue was subjected to flash chromatography on silica gel, unless otherwise noted. The yields of the cross-coupling are listed in FIG. 11. To form cross-coupled product 6a, 1-(4-(furan-2-yl)phenyl)ethanone, the general procedure was followed using 4-bromoacetophenone (0.200 g, 1.00 mmol), MIDA boronate 2a (0.336 g, 1.51 mmol), and K3PO4-H2O (1 .733 g, 7.53 mmol). The product was eluted with 100% CH2CI2 to afford 6a as a pale yellow solid (0.183 g, 98%). TLC (hexanes:EtOAc 6:1) Rf = 0.25, visualized by UV (254 and 366 nm) and KMnO4. 1H-NMR (500 MHz, CDCl3) δ 7.98 (d, / = 8 Hz, 2H), 7.75 (d, / = 8 Hz, 2H), 7.53 (d, / = 2 Hz, 1 H), 6.80 (d, / = 3.5 Hz, 1 H), 6.52 (dd, / = 3.5, 2 Hz, 1 H), 2.61 (s, 3H). 13C-NMR (125 MHz, CDCl3) δ 197.3, 152.8, 143.2, 135.5, 134.8, 128.9, 123.5, 1 12.0, 107.4, 26.5. HRMS (Cl +) Calculated for C12H11O2 (M + H) + : 187.0759; Found: 187.0756. IR (thin film, cm-1) 31 10, 1669, 1609, 1475, 1416, 1378, 1299, 1223, 1 120, 1079, 1020, 1010, 905, 839, 821.
References:
WO2010/36921,2010,A2 Location in patent:Page/Page column 65-66
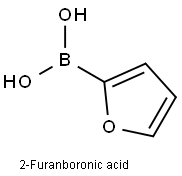
13331-23-2
352 suppliers
$13.43/250mgs:
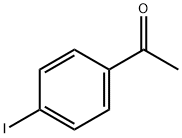
13329-40-3
271 suppliers
$6.00/5g

35216-08-1
24 suppliers
inquiry

13331-23-2
352 suppliers
$13.43/250mgs:

99-90-1
474 suppliers
$6.00/25g

35216-08-1
24 suppliers
inquiry