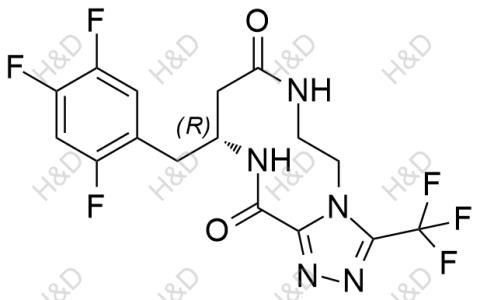
產(chǎn)品名稱:西格列汀雜質(zhì)24
英文名稱:Sitagliptin Impurity 24
貨號(hào):S007032
CAS號(hào):2088771-61-1
分子式:C16H13F6N5O2
分子量:421.3
純度:>95%
用途:供新藥研究及實(shí)驗(yàn)使用
隨貨:提供COA��,質(zhì)譜���,氫譜�����,HPLC�。
自主藥物雜質(zhì)對(duì)照品品牌H&D,提供藥物雜質(zhì)�、小分子化合物合成定制及制備分離等服務(wù),產(chǎn)品包括藥物標(biāo)準(zhǔn)品��、藥物雜質(zhì)對(duì)照品�、特色中間體、分析檢測(cè)以及化合物定制合成服務(wù)���。匯集超數(shù)萬(wàn)種雜質(zhì)對(duì)照品和標(biāo)準(zhǔn)品�����,已完成數(shù)百種特色中
自主藥物雜質(zhì)對(duì)照品品牌H&D�,提供藥物雜質(zhì)�、小分子化合物合成定制及制備分離等服務(wù),產(chǎn)品包括藥物標(biāo)準(zhǔn)品��、藥物雜質(zhì)對(duì)照品����、特色中間體�����、分析檢測(cè)以及化合物定制合成服務(wù)�。匯集超數(shù)萬(wàn)種雜質(zhì)對(duì)照品和標(biāo)準(zhǔn)品��,已完成數(shù)百種特色中間體定制合成��。種類齊全����,涵蓋98%以上前沿仿制藥項(xiàng)目;圖譜齊全�,COA、氫譜�、碳譜、質(zhì)譜�、液相、紫外��、紅外����、TGA、定量氫譜����、二維譜應(yīng)有盡有;
現(xiàn)貨多����、貨期快、價(jià)格省���、質(zhì)量?jī)?yōu)����。
風(fēng)帶來(lái)了涼爽也帶來(lái)了思念�,我們的產(chǎn)品期待著與您見面,您不勇敢一點(diǎn)�����,怎能知道雜質(zhì)購(gòu)買如此簡(jiǎn)便����!
快來(lái)聯(lián)系我吧!
聯(lián)系人: 劉先生
手機(jī)(微信):18126517264
QQ:2850476883
公司官網(wǎng):www.hdbiochemical.com,可供您查詢更多熱銷雜質(zhì)結(jié)構(gòu)��!
OS:歡迎咨詢��,真誠(chéng)地期待與您的合作��,服務(wù)好每位客戶是我們的榮幸�!
譜、碳譜����、質(zhì)譜、液相�����、紫外�����、紅外�、TGA、定量氫譜��、二維譜應(yīng)有盡有�����;
現(xiàn)貨多、貨期快����、價(jià)格省����、質(zhì)量?jī)?yōu)。
風(fēng)帶來(lái)了涼爽也帶來(lái)了思念�����,我們的產(chǎn)品期待著與您見面���,您不勇敢一點(diǎn)�,怎能知道雜質(zhì)購(gòu)買如此簡(jiǎn)便��!
快來(lái)聯(lián)系我吧��!
聯(lián)系人: 劉先生
手機(jī)(微信):18126517264
QQ:2850476883
公司官網(wǎng):www.hdbiochemical.com����,可供您查詢更多熱銷雜質(zhì)結(jié)構(gòu)!
OS:歡迎咨詢,真誠(chéng)地期待與您的合作�����,服務(wù)好每位客戶是我們的榮幸��!
關(guān)鍵字: 西格列汀雜質(zhì);雜質(zhì);對(duì)照品;現(xiàn)貨;恒豐萬(wàn)達(dá);
恒豐萬(wàn)達(dá)醫(yī)藥科技有限公司致力于為醫(yī)藥科學(xué)研究領(lǐng)域提供產(chǎn)品和服務(wù)�����。產(chǎn)品主要包括醫(yī)藥行業(yè)標(biāo)準(zhǔn)品�,雜質(zhì)對(duì)照品,植物單體及對(duì)照品�,進(jìn)口試劑及耗材等。公司與眾多 的試劑公司�、藥企、研發(fā)機(jī)構(gòu)及高校等建立了良好的合作關(guān)系����,為客戶提供咨詢,定制��,代理進(jìn)出口等服務(wù)�。我們通過快速的交貨,合理的價(jià)格��,完善的技術(shù)支持和售后服務(wù),與國(guó)內(nèi)外眾多客戶建立了穩(wěn)定的業(yè)務(wù)往來(lái)����。