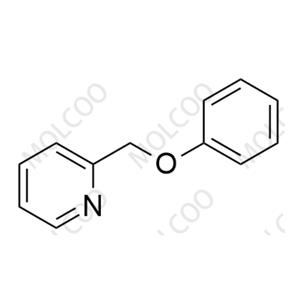
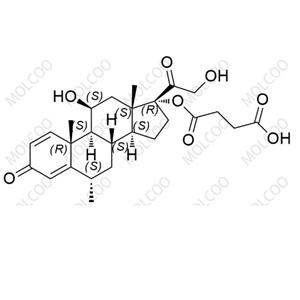
Bisacodyl Impurity 1 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-01-22 |
Product Details
| Product Name: Bisacodyl Impurity 1 | CAS No.: 104294-19-1 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/01/22 |

Detailed Purpose Description:
Pharmaceutical Research and Development: In the drug development stage, Bisacodyl Impurity Reference Standards are used to assess the impurity content in new drugs, ensuring their safety and effectiveness. By conducting quantitative analysis of Bisacodyl impurities, researchers can optimize production processes, reduce impurity generation, and improve drug quality.
Quality Control: During drug production, Bisacodyl Impurity Reference Standards serve as a key tool for quality control, used to detect whether the impurity content in finished drugs meets specified standards. This helps ensure that each batch of produced drugs achieves the expected purity and quality.
Drug Testing: When conducting drug approval and testing, regulatory agencies use Bisacodyl Impurity Reference Standards to verify the accuracy of detection methods and results for impurities in drugs. This helps safeguard public drug safety and prevent unqualified drugs from entering the market.
Stability Studies: In drug stability studies, Bisacodyl Impurity Reference Standards are used to evaluate impurity generation in drugs under different storage conditions. This helps determine the shelf life and storage conditions of drugs, ensuring they maintain stable quality within their validity period.
Regulatory Compliance: The use of Bisacodyl Impurity Reference Standards helps pharmaceutical companies comply with relevant domestic and international drug regulations and quality standards, ensuring drugs meet market requirements.

Company Profile Introduction
You may like
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake




 China
China