| Company Name: |
Mainchem Co., Ltd.
|
| Tel: |
+86-0592-6210733 |
| Email: |
sale@mainchem.com |
| Products Intro: |
Product Name:ZINC CHLORATE
CAS:10361-95-2
|
|
| | ZINC CHLORATE Basic information |
| Product Name: | ZINC CHLORATE | | Synonyms: | chloricacid,zincsalt;ZINC CHLORATE;Zinkchlorat;Bischloric acid zinc salt;zinc,dichlorate | | CAS: | 10361-95-2 | | MF: | Cl2O6Zn | | MW: | 232.29 | | EINECS: | 233-804-7 | | Product Categories: | | | Mol File: | 10361-95-2.mol | 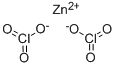 |
| | ZINC CHLORATE Chemical Properties |
| Melting point | decomposes at 60℃ [HAW93] | | density | 2.150 | | form | yellow hygroscopic crystals | | color | yellow hygroscopic crystals, crystalline | | Water Solubility | mol/100mol H2O: 59.19 (0°C), 66.52 (18°C), 75.44 (55°C); solid phase, Zn(ClO3)2 · 6H2O (0°C), Zn(ClO3)2 ·4H2O (18°C, 55°C) [KRU93]; soluble alcohol, glycerol, ether [HAW93] | | EPA Substance Registry System | Zinc chlorate (10361-95-2) |
| | ZINC CHLORATE Usage And Synthesis |
| Chemical Properties | Colorless to yellowish crystals. Deliquescent,
decomposes at 60C. Soluble in
water, alcohol, glycerol, and ether | | General Description | A white crystalline solid. May explode under prolonged exposure to heat or fire. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | ZINC CHLORATE is an oxidizing agent. Forms flammable mixtures with combustible materials; these mixtures may be explosive if the combustible material is finely divided. Contact with concentrated sulfuric acid can cause fires or explosions. Heating with a moist dibasic organic acid liberates chlorine dioxide and carbon dioxide [Bretherick 1979. p. 100] mixtures with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive [Bretherick 1979. p. 806]. A combination with finely divided aluminum can explode by heat, percussion, or friction [Mellor 2:310 1946-47]. A mixture with charcoal (or other finely divided organic material) may ignite or explode. Ignition or explosion may also be caused by friction or shock [U.S. Army Ordnance Safety Manual 1951]. | | Health Hazard | Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard. | | Safety Profile | A powerful oxidizer.
Probably a skin, eye, and mucous membrane
irritant. The tetrahydrated salt explodes at
60°C. Explosive reaction with copper(II)
sulfide. Can react violently with Al, Sb2S3,
As, C, charcoal, Cu, MnO2, metal sulfides,
dibasic organic acids, organic matter, P, S,
H2SO4. Incandescent reaction with
antimony(III) sulfide, arsenic(III) sulfide,
tinpI) sulfide, tin(IV> sulfide. When heated
to decomposition it emits toxic fumes of CT
and ZnO. See also CHLORATES and
ZINC COMPOUNDS. |
| | ZINC CHLORATE Preparation Products And Raw materials |
|