| Company Name: |
BioBioPha Co., Ltd.
|
| Tel: |
0871-65217109 13211707573; |
| Email: |
y.liu@mail.biobiopha.com |
| Products Intro: |
CAS:639-36-1
Purity:>97.0% Package:5mg Remarks:BBP03110
|
|
| | (+)-Polyneuridine Basic information |
| Product Name: | (+)-Polyneuridine | | Synonyms: | 17-Hydroxysarpagane-16-carboxylic acid methyl ester;Rhazin;SARPAGAN-16-CARBOXYLIC ACID17-HYDROXY-,METHYL ESTER;Akuammidine;Rhazine;(+)-Polyneuridine | | CAS: | 639-36-1 | | MF: | C21H24N2O3 | | MW: | 352.43 | | EINECS: | | | Product Categories: | | | Mol File: | 639-36-1.mol | 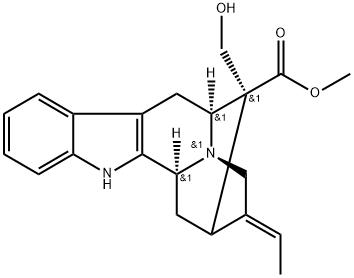 |
| | (+)-Polyneuridine Chemical Properties |
| Melting point | 243-246℃ | | Boiling point | 513.0±50.0 °C(Predicted) | | density | 1.34±0.1 g/cm3(Predicted) | | pka | 14.79±0.10(Predicted) |
| | (+)-Polyneuridine Usage And Synthesis |
| Uses | Akuammidine, isolated from the seeds of Picralima nitida, shows a preference for μ-opioid binding sites with Ki values of 0.6, 2.4 and 8.6 μM at μ-, σ- and κ-opioid binding sites, respectively. Akuammidine possesses anti-inflammatory and anti-asthmatic properties[1][2]. | | IC 50 | κ Opioid Receptor/KOR | | References | [1] Yuanyuan Hou, et al. Microfractionation Bioactivity-Based Ultra Performance Liquid Chromatography/Quadrupole Time-Of-Flight Mass Spectrometry for the Identification of Nuclear factor-κB Inhibitors and β2 Adrenergic Receptor Agonists in an Alkaloidal Extract of the Folk Herb Alstonia Scholaris. J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Nov 1;908:98-104. DOI:10.1016/j.jchromb.2012.10.004
[2] J R Menzies, et al. Opioid Activity of Alkaloids Extracted From Picralima Nitida (Fam. Apocynaceae). Eur J Pharmacol. 1998 May 29;350(1):101-8. DOI:10.1016/s0014-2999(98)00232-5 |
| | (+)-Polyneuridine Preparation Products And Raw materials |
|