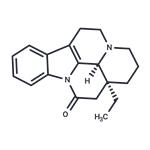
- Vinburnine
-
- $80.00 / 100mg
-
2024-11-19
- CAS:4880-88-0
- Min. Order:
- Purity: 99.86%
- Supply Ability: 10g
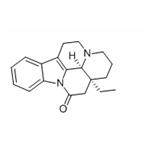
- (-)-EBURNAMONINE
-
- $1.00 / 1KG
-
2024-08-08
- CAS:4880-88-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20000KG
|
| | (-)-EBURNAMONINE Basic information |
| | (-)-EBURNAMONINE Chemical Properties |
| Melting point | 174-177 °C(lit.) | | alpha | D25 -102° (chloroform) (Clauder); D -100° (c = 0.783 in chloroform) (Cartier) | | Boiling point | 436.16°C (rough estimate) | | density | 1.34 | | refractive index | 1.5600 (estimate) | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly), Methanol (Slightly, Heated) | | form | White to slightly yellow crystalline powder. | | pka | 8.13±0.40(Predicted) | | color | White | | Merck | 13,3520 | | LogP | 3.790 (est) | | CAS DataBase Reference | 4880-88-0(CAS DataBase Reference) |
| Safety Statements | 22-24/25 | | WGK Germany | 2 | | RTECS | YY8575570 | | F | 8-10 |
| | (-)-EBURNAMONINE Usage And Synthesis |
| Chemical Properties | white to slightly yellow crystalline powder | | Originator | Euburnamonine ,Shanghai Lansheng
Corporation | | Uses | Cerebral vasodilatator | | Uses | (-)-Eburnamonine shows protective effects on mice from the lethal consequences of hypoxia. | | Definition | ChEBI: Eburnamonine is an alkaloid. | | Manufacturing Process | 200 g (0.087 moles) of cis-1-ethyl-1-(2'-hydroxy-2'-carboxyethyl)-
1,2,3,4,6,7,12,12b-octahydro-indolo[2,3-a]quinolizine were suspended in 100
ml of xylene. 12 g of supported silver carbonate (a silver carbonate-Celite
reagent, containing 50% of silver carbonate) were added to the suspension,
and the system was boiled for 8 hours under a nitrogen atmosphere, with
constant stirring. Thereafter the hot solution was filtered, washed with 3 x 30
ml to 5% sodium carbonate solution, dried over magnesium sulfate, and
evaporated under reduced pressure. 1.05 g of a solid residue, which was a
mixture of racemic vincamone and racemic vincanol, was obtained. 40 ml of
ethyl ether were added to the mixture, and the insoluble crystals were filtered
off. The crystals were recrystallized from methanol, to yield 0.5 g of (+/-)-
eburnamonine (vincamone). Yield: 29.5%. MP: 201°-202°C. The value was
identical with the literature value (J. Mokry et aI.: CoII. Czech, Chem. Comm.
28, 1309, 1963). The etheral mother liquor was processed by preparative
layer chromatography (adsorbent: Kieselgel PF 254+366 , developing agent: a
14:2 mixture of benzene and methanol). The obtained substance was
recrystallized from ether to yield 0.2 g (16%) of (+/-)-vincanol (eburnamine).
MP: 163-164°C, under decomposition. The IR spectrum of the obtained
substance was identical with that of the authentic sample.
Resolution of (+/-)-vincamone:
10.0 g (0.0342 moles) of (+/-)-vincamone and 12.2 g (0.0342 moles) of (-)-
dibenzoyl tartaric acid were dissolved in 150 ml of dichloromethane, the
solution was seeded with crystalline (-)-vincamone-(-)-dibenzoyl-tartarate,
and the mixture was left to stand. The separated crystals were filtered off,
dissolved in dimethylformamide, and the pH of the solution was adjusted to 9 with aqueous ammonia. The separated substance was filtered off, washed with
water, and dried. This way 4.2 g (84%) of (-)-vincamone were obtained; MP:
-176°C; α D 20 = -96°. The mother liquor of resolution was evaporated and (+)-
vincamone was separeted as described above. The obtained substance had
MP: 173°-176°C.; α D 20 = +96°. | | Therapeutic Function | Cerebrotonic |
| | (-)-EBURNAMONINE Preparation Products And Raw materials |
|