| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Email: |
sales8178@energy-chemical.com |
| Products Intro: |
Product Name:(+)-CaMphene
CAS:5794-03-6
Purity:technical grade, 80% Package:100G
|
| Company Name: |
T&W GROUP
|
| Tel: |
021-61551611 13296011611 |
| Email: |
contact@trustwe.com |
| Products Intro: |
Product Name:(+)-CAMPHENE
CAS:5794-03-6
Purity:98% Package:按需包裝
|
(+)-CAMPHENE manufacturers
- (+)-CaMphene
-

- $1.00 / 1KG
-
2020-01-08
- CAS: 5794-03-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20T
|
| | (+)-CAMPHENE Basic information |
| Product Name: | (+)-CAMPHENE | | Synonyms: | 2,2-dimethyl-3-methylene-,(1R)-Bicyclo[2.2.1]heptane;2-dimethyl-3-methylene-(1theta)-bicyclo[2.2.1]heptan;Camphene, (1R,4S)-(+)-;d-Camphene;FEMA 2229;2��,2-Dimethyl-3-methylenenorborhane;(+)-CAMPHENE 80+% FCC;(+)-CAMPHENE, TECH., 80% | | CAS: | 5794-03-6 | | MF: | C10H16 | | MW: | 136.23 | | EINECS: | 227-336-2 | | Product Categories: | | | Mol File: | 5794-03-6.mol | 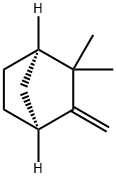 |
| | (+)-CAMPHENE Chemical Properties |
| Melting point | 40-50 °C(lit.) | | Boiling point | 159-160 °C(lit.) | | density | 0.839 g/mL at 25 °C(lit.) | | refractive index | 1.4562 (estimate) | | Fp | 98 °F | | solubility | Benzene (Slightly), Chloroform (Slightly), | | form | Solid | | color | White to Off-White Waxy | | Odor | at 10.00 % in dipropylene glycol. fresh herbal woody fir camphor | | Odor Type | woody | | optical activity | [α]20/D +16°, c = 4 in ethanol | | LogP | 4.220 | | EPA Substance Registry System | Bicyclo[2.2.1]heptane, 2,2-dimethyl-3-methylene-, (1R,4S)- (5794-03-6) |
| Hazard Codes | F | | Risk Statements | 11 | | Safety Statements | 16-33 | | RIDADR | UN 1325 4.1/PG 2 | | WGK Germany | 3 |
| | (+)-CAMPHENE Usage And Synthesis |
| Physical properties | Camphene is a colorless, crumbly crystalline
solid with a camphor-like odor. It has a tendency
to sublime. | | Uses | (+)-Camphene is part of a mixture which is responsible for the anti-inflammatory effect of essential oil from Citrus aurantium L. var. amara Engl. | | Production Methods | The extraction of camphene by distillation of
turpentine is now hardly used in industry. Instead camphene is produced from a-pinene. | | Definition | ChEBI: A camphene (2,2-dimethyl-3-methylenebicyclo[2.2.1]heptane) that has R configuration at position 1 and S configuration at position 4. | | Chemical Reactivity | Camphene is stable in air and light. At elevated temperatures, in the presence of oxygen, it undergoes autoxidation to camphenilone. Peracids attack the double bond, giving camphene oxide. Catalytic hydrogenation gives the saturated hydrocarbon isocamphane. | | Biochem/physiol Actions | Taste at 100 ppm |
| | (+)-CAMPHENE Preparation Products And Raw materials |
|