|
|
| | 3,4,5-Trifluorophenylboronic acid Basic information | | Uses |
| Product Name: | 3,4,5-Trifluorophenylboronic acid | | Synonyms: | AKOS BRN-0138;3,4,5-TRIFLUOROPHENYLBORONIC ACID;3,4,5-TRIFLUOROBENZENEBORONIC ACID;3,4,5-Trifluorobenzeneboronic acid 97%;3,4,5-Trifluorobenzeneboronicacid97%;3,4,5-TRIFLUOROPHENYLBORONIC ACID MIN 96% HPLC;3,4,5-Trifluorophenylboronic Aicd;Boronic acid, B-(3,4,5-trifluorophenyl)- | | CAS: | 143418-49-9 | | MF: | C6H4BF3O2 | | MW: | 175.9 | | EINECS: | 604-355-8 | | Product Categories: | Piperidines;Aryl;Fluorinated;Organoborons;Substituted Boronic Acids;Fluorin-contained phenyl boronic acid series;Boronic Acid;blocks;BoronicAcids;Boronic Acid series;Fluoro-Aromatics;Boronic Acids and Derivatives;Boronic Acids;Boronic Acids | | Mol File: | 143418-49-9.mol | 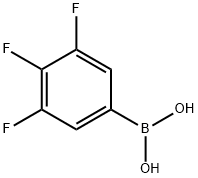 |
| | 3,4,5-Trifluorophenylboronic acid Chemical Properties |
| Melting point | 290-295 °C (lit.) | | Boiling point | 263.6±50.0 °C(Predicted) | | density | 1,087g/cm | | refractive index | 1,423-1,425 | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | 18.43g/l | | pka | 6.54±0.11(Predicted) | | form | Powder | | color | Tan | | BRN | 7371914 | | InChI | InChI=1S/C6H4BF3O2/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1-2,11-12H | | InChIKey | UHDDEIOYXFXNNJ-UHFFFAOYSA-N | | SMILES | B(C1=CC(F)=C(F)C(F)=C1)(O)O | | CAS DataBase Reference | 143418-49-9(CAS DataBase Reference) |
| | 3,4,5-Trifluorophenylboronic acid Usage And Synthesis |
| Uses | Reactant involved in:
? Preparation of phenylboronic catechol esters and determination of Lewis acidity
? Synthesis of benzopyranone derivatives as GABAA receptor modulators
? Synthesis of multisubstituted olefins and conjugate dienes
? Suzuki cross-coupling reactions
? Preparation of fluorinated aromatic poly(ether-amide)s | | Description | 3,4,5-Trifluorophenylboronic acid is an arylboronic acid compound used in organic synthesis and as a reaction reagent. It can be used as a catalyst to catalyse the direct amidation of amino acid derivatives and the direct polycondensation of carboxylic acids and amines; it can also be used in the formation of new 1:1 co-crystals with urea C6H4BF3O2-CH4N2O components. | | Chemical Properties | Tan powder | | Uses | Reactant involved in:
- Preparation of phenylboronic catechol esters and determination of Lewis acidity
- Synthesis of benzopyranone derivatives as GABAA receptor modulators
- Synthesis of multisubstituted olefins and conjugate dienes
- Suzuki cross-coupling reactions
- Preparation of fluorinated aromatic poly(ether-amide)s
| | Uses | suzuki reaction | | Uses | Intermediates of Liquid Crystals | | Synthesis | Add 42mmol (10.08g) of magnesium chips into a 1000mL four-necked flask, add 2 pieces of iodine, and then add 200mL of anhydrous 2-methyltetrahydrofuran, heat the temperature to 50°C, and then slowly let it cool down to 40°C. And then drip 4-5mL of 3,4,5-trifluorobromobenzene 84.4g (0.4mol) and 50mL 2-methyltetrahydrofuran mixed solution, trigger the Grignard reaction, the temperature will rise rapidly after the reaction is initiated, use The temperature is lowered to 10-15°C in an ice-water bath, and a mixture (1) composed of 3,4,5-trifluorobromobenzene 84.4g (0.4mol) and 50mL 2-methyltetrahydrofuran (1), trimethyl borate is added dropwise. A mixture of 44 grams (0.42 mol) of ester and 50 mL of 2-methyltetrahydrofuran (2).The speed of the mixed solution (1) and the mixed solution (2) is kept the same, the dropping speed is controlled, and the dropping temperature is kept at 10-15°C . After the dropping is completed, the reaction is continued at room temperature for 8 hours.After the reaction is over, the reaction solution is slowly poured into dilute hydrochloric acid, and after the addition is complete, stir for 0.5-1.5h, separate the liquids, and then extract the aqueous phase twice, combine the organic phases, dry with anhydrous sodium sulfate, spin dry, and Recrystallization of ethyl chloride gave 3,4,5-Trifluorophenylboronic acid with a yield of 78% and a content of 99.6%. |
| | 3,4,5-Trifluorophenylboronic acid Preparation Products And Raw materials |
|