- Tetracycline
-

- $10.00 / 1KG
-
2024-11-28
- CAS:60-54-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Tetracycline
-
- $33.00 / 100mg
-
2024-11-18
- CAS:60-54-8
- Min. Order:
- Purity: 92.65%
- Supply Ability: 10g
- Tetracycline
-
- $33.00 / 100mg
-
2024-11-18
- CAS:60-54-8
- Min. Order:
- Purity: 92.65%
- Supply Ability: 10g
Related articles - Mechanism of action of Tetracycline
- Tetracycline was first described in 1953 and was prepared from chlortetracycline at Lederle Laboratories, and was also indepen....
- Mar 17�����,2022
|
| | Tetracycline Basic information |
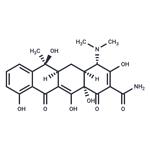
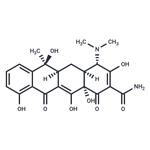
| Product Name: | Tetracycline | | Synonyms: | 12,12a-pentahydroxy-6-methyl-1,11-dioxo-1;4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-4-(dimethylamino)-4;6-methyl-1,11-dioxy-2-naphthacenecarboxamide;abramycin;bio-tetra;bristaciclinalpha;cefracyclinesuspension;ciclibion | | CAS: | 60-54-8 | | MF: | C22H24N2O8 | | MW: | 444.43 | | EINECS: | 200-481-9 | | Product Categories: | Peptide Synthesis/Antibiotics;Intermediates & Fine Chemicals;Pharmaceuticals;Antibacterial;Antibiotics;Antibiotics A to;Antibiotics by Application;Antibiotics T-ZAntibiotics;Chemical Structure Class;Genetic Marker SelectionAntibiotics;Interferes with Protein SynthesisSpectrum of Activity;L - ZAntibiotics;Mechanism of Action;Tetracyclines;Antibiotic Explorer;Inhibitors;60-54-8 | | Mol File: | 60-54-8.mol | 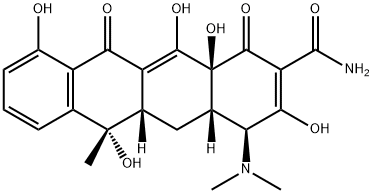 |
| | Tetracycline Chemical Properties |
| Melting point | 175-177 °C(lit.) | | alpha | D25 -257.9° (0.1N HCl); D25 -239° (methanol) | | Boiling point | 554.44°C (rough estimate) | | density | 1.3809 (rough estimate) | | refractive index | 1.6500 (estimate) | | storage temp. | 2-8°C | | solubility | 95% ethanol: soluble12.5mg/mL | | form | powder | | pka | pKa (50% aq DMF): 8.3, 10.2(at 25℃) | | color | yellow to yellow orange | | optical activity | [α]25/D -239° in methanol (Specific rotation ) &_& [α]25/D -257.9°, c = 0.1 M HCl mol (Specific rotation ) | | Water Solubility | Limited solubility in water. Soluble in 1M HCl with heating. | | Merck | 13,9271 | | BRN | 2230417 | | BCS Class | 3 | | Stability: | Hygroscopic | | InChIKey | OFVLGDICTFRJMM-WESIUVDSSA-N | | CAS DataBase Reference | 60-54-8(CAS DataBase Reference) | | NIST Chemistry Reference | Tetracycline(60-54-8) | | EPA Substance Registry System | Tetracycline (60-54-8) |
| | Tetracycline Usage And Synthesis |
| Description | Tetracycline is an antibiotic that has been utilized in
disease management situations in which SmR strains of
E. amylovora or P. syringae already exist. However, tetracycline
does not appear to be as effective as streptomycin in
reducing blossom populations of E. amylovora (17). Additionally,
strains of P. syringae with resistance to tetracycline
have been isolated from pear orchards in Oregon
and Washington (18), suggesting that resistance to this
antibiotic will probably develop in orchards where it is
applied. | | Chemical Properties | Crystalline Solid | | Chemical Properties | Tetracycline trihydrate is a white crystalline substance. | | Originator | Tetracyn,Pfizer,US,1953 | | Uses | antibactierial | | Uses | Antibiotic substance produced by Streptomyces spp. Antiamebic; antibacterial; antirickettsial | | Uses | Tetracycline is a broad spectrum polyketide antibiotic. It is used clinically to treat bacterial infections such as Rocky Mountain spotted fever, typhus fever, tick fevers, Q fever, and Brill-Zinsser disease. It is used to treat upper respiratory infections and acne. It is used to study multidrug resistance as well as potential side effects such as acute pancreatitis. | | Uses | Tetracycline is a linear, tetracyclic, broad spectrum antibiotic first prepared chemically by dechlorination of chlortetracycline and subsequently isolated from several Streptomyces species. Tetracycline has broad spectrum antibacterial and antiprotozoan activity, and acts by binding to the 30S and 50S ribosomal subunits blocking protein synthesis. Tetracycline is a pigment and, like many pigments, is degraded by light, oxygen, trace metal ions and pH variations. The purity of tetracycline is often variable, with significant levels of degradation products. | | Uses | antibacterial, antiamebic, antirickettsial | | Indications | Tetracycline is often the first antibiotic prescribed. It is the least expensive,
has few side effects, and is well tolerated for longer periods of time. Tetracycline
is effective in low doses because high concentrations are achieved within
sebaceous follicles, especially when inflammation is present. | | Definition | ChEBI: Tetracycline is a broad-spectrum polyketide antibiotic produced by the Streptomyces genus of actinobacteria. It has a role as an antimicrobial agent, an antibacterial drug, an antiprotozoal drug, a protein synthesis inhibitor and an Escherichia coli metabolite. It is a tertiary alpha-hydroxy ketone and a member of tetracyclines. It is a conjugate acid of a tetracycline(1-) and a tetracycline zwitterion. | | Manufacturing Process | Tetracycline is usually prepared by the catalytic dechlorination of
chlortetracycline as described in US Patents 2,699,054 and 3,005,023, or
obtained directly by fermentation of Streptomyces aureofaciens or
Streptomyces viridifaciens according to US Patents 2,712,517, 2,734,018,
2,886,595 and 3,019,173. The purification of tetracycline produced by either
route is described in US Patent 3,301,899.
The production of tetracycline by catalytic dechlorination is described in US
Patent 2,699,054 as follows: Pure chlortetracycline (4.8 grams) was
suspended in 100 ml of methanol and sufficient anhydrous dioxane was added
to completely dissolve the product. To the solution was added 0.5 gram of 5%
palladium-on-charcoal catalyst. The mixture was placed in a conventional
hydrogenation apparatus and subjected to a pressure of 50 psi of hydrogen
while being agitated.
After the initial drop in pressure due to the absorption of gas by the catalyst
and the solvent, there was a steady drop in pressure due to the
hydrogenation of the antibiotic. After approximately 1 mol of hydrogen had
been absorbed, no further reaction was observed. This occurred after about 2
hours. The catalyst was filtered and washed with boiling methanol and boiling
dioxane. The solution gave a positive test for chloride ion when treated with
silver nitrate solution. It also possessed a strongly acidic reaction
demonstrating the release of the nonionic chlorine in the form of hydrogen
chloride. A bioassay of the crude product in solution indicated a potency of
approximately 580 μg/mg with oxytetracycline as the standard at a potency of
1,000 μg/mg. The solution was concentrated under vacuum at room
temperature and the residual liquid was dried from the frozen state under
vacuum. 3.1 grams of bright yellow amorphous tetracycline hydrochloride was
obtained.
This product may be converted to tetracycline per se by redissolving it in
water, carefully neutralizing it to pH 4.5 with dilute sodium hydroxide, and
recovering the product by drying the solution. | | Brand name | Achrocidin;Achromycin v;Achromycin y;Apo-tetra;Cyclopar;Decycline;Double-t;Gt-250;Hosta-500;Medicycline;Muracine;Mysteclin-f;Nasopomada;Neo-tetrine;Nor-tet;Novotetra;Retet;Robitet;Sk-tetracycline;Sumycin;Tepcycline;Teropicycline;Tetrabotic;Tetracaps;Tetracyn;Tetralan;Tetram;Tetrex;Tetrpsol;Wintracin. | | Therapeutic Function | Antibacterial | | World Health Organization (WHO) | The first tetracycline antibiotic, chlortetracycline, was introduced
in 1948 and subsequently several semisynthetic derivatives have been used as
antibacterial, antiamoebic and antirickettsial agents. All tetracyclines accumulate in
the developing bones and teeth of the foetus and young children which can result
in retarded bone growth and dental staining. Preparations intended specifically for
children have been withdrawn in some countries, whereas in others warnings are
required on the label advising against administration of tetracyclines to young
children and pregnant women. Non-paediatric dosage forms of tetracycline remain
in the WHO Model List of Essential Drugs. | | Antimicrobial activity | It is also active against V. cholerae, chlamydiae,
rickettsiae and spirochetes. | | General Description | Chemical studies on chlortetracycline revealed that controlledcatalytic hydrogenolysis selectively removed the 7-chloro atom and so produced tetracycline (Achromycin,Cyclopar, Panmycin, Tetracyn). This process was patentedby Conover in 1955. Later, tetracycline was obtainedfrom fermentations of Streptomyces spp., but the commercialsupply still chiefly depends on hydrogenolysis of chlortetracycline.
Tetracycline is 4-dimethyl amino-1,4,4a,5,5a,6,11,12aoctahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide. It is a bright yellow, crystallinesalt that is stable in air but darkens on exposure tostrong sunlight. Tetracycline is stable in acid solutions witha pH above 2. It is somewhat more stable in alkaline solutionsthan chlortetracycline, but like those of the other tetracyclines,such solutions rapidly lose potency. One gram ofthe base requires 2,500 mL of water and 50 mL of alcohol todissolve it. The hydrochloride salt is used most commonly inmedicine, though the free base is absorbed from the GI tractabout equally well. One gram of the hydrochloride salt dissolvesin about 10 mL of water and in 100 mL of alcohol.Tetracycline has become the most popular antibiotic of itsgroup, largely because its plasma concentration appears to behigher and more enduring than that of either oxytetracyclineor chlortetracycline. Also, it is found in higher concentrationin the spinal fluid than the other two compounds. | | Pharmaceutical Applications | A fermentation product of Streptomyces aureofaciens, also produced
from chlortetracycline. Available as the hydrochloride
for oral and topical use. | | Pharmacokinetics | Oral absorption: c.75%
Cmax 500 mg oral: 2–4 g/L
Plasma half-life: 8.5 h
Volume of distribution: c.1.3 L/kg
Plasma protein binding: c.50–60%
Absorption
When taken with food, absorption is reduced by approximately
50%. Steady-state plasma concentrations of 4–5
mg/L occur after oral doses of 500 mg every 6 h. Women
appear to produce higher concentrations than men. Divalent
and trivalent cations such as calcium and aluminum present
in antacids and milk interfere with absorption through
chelation, as does ferrous sulfate. H2-receptor antagonists,
by raising gastric pH, also interfere with absorption through
impaired drug dissolution. Despite the effect of gastric
pH, oral absorption is not affected in elderly patients with
achlorhydria.
Distribution
Tetracycline is widely distributed in the body tissues. In
particular, it penetrates well into the prostate, uterus, ovary
and bladder, and also appears to be preferentially taken
up by the gastrointestinal tract. It is also detectable within
reticuloendothelial cells of the liver, spleen and bone
marrow.
Protein binding is reduced in states of malnutrition. It is
also bound to bone, dentine and tooth enamel of unerupted
teeth. Sputum concentrations of 0.4–2.6 mg/L have been
detected after 250 mg oral dosage every 8 h. Maxillary sinus
secretions and bronchial mucosal tissue have concentrations
comparable to those of serum.
CSF penetration is poor, but increases with meningeal
inflammation. It crosses the placenta readily to enter the fetal
circulation, where it achieves 25–75% of the maternal plasma
concentration. It is also present in breast milk.
Metabolism
A small amount is metabolized to 4-epitetracycline.
Excretion
Tetracycline is largely eliminated unchanged by glomerular
filtration, with more than 50% excreted within 24 h after
oral administration. This rises to approximately 70% following
parenteral administration. Urinary concentrations of
300 mg/L occur within the first 2 h and persist for up to 12 h.
Urinary excretion is enhanced in alkaline urine. Renal clearance
is reduced in severe protein calorie malnutrition, possibly
through reduced glomerular filtration. It accumulates in
the presence of renal failure and is only slowly removed by
hemodialysis and minimally by peritoneal dialysis.
The bile is an important route of excretion, accounting for
about one-third of the dose. Biliary concentrations may be
10–25 times those found in serum. Impaired hepatic function
or biliary obstruction leads to an increase in blood levels. | | Clinical Use | Along with doxycycline
it is one of the most commonly used tetracyclines. | | Side effects | The gastrointestinal side effects common to the group are the
most frequent cause of intolerance. Metallic taste and glossitis
are less burdensome than diarrhea. Antibiotic-associated
enterocolitis caused by Clostridium difficile toxin and staphylococcal
enterocolitis have been reported. Steatorrhea and
acute pancreatitis has also been described. Irritation and
ulceration of the esophagus has occurred with local impaction
of the drug. C. albicans overgrowth is common and may
result in symptomatic oral or vaginal candidiasis and occasionally
candida diarrhea.
Hypersensitivity reactions include contact dermatitis, urticaria,
facial edema and asthma. Anaphylaxis is rare. A lupus
syndrome has been reported, but its cause is uncertain.
Photosensitivity can be severe and cause vesiculation, desquamation
and onycholysis. The Jarisch–Herxheimer reaction
has been observed in the treatment of syphilis, louse-borne
relapsing fever, leptospirosis, brucellosis and tularemia.
Deposition in deciduous teeth and bone (where it may temporarily
inhibit growth) is of continuing concern. Between
3% and 44% of administered tetracycline is incorporated in
the inorganic phase of bone, which may become visibly discolored
and fluoresce. Concentrations as high as 290 mg/g
have been recorded in bone in those on long-term tetracycline
treatment for acne.
Existing renal insufficiency may be aggravated and is probably
related to the antianabolic effect of this class of drugs;
interference with protein synthesis places an additional burden
on the kidney from amino acid metabolism. Acute renal failure
may occur and can be aggravated by drug-induced diarrhea.
Dehydration and salt loss from diuretic therapy may aggravate
nephrotoxicity. Methoxyflurane and tetracycline in combination
may be synergistically nephrotoxic.
An uncommon but serious adverse reaction is acute fatty
liver, which may be complicated by renal insufficiency and
electrolyte abnormalities. This is most likely to occur with highdose
intravenous administration, especially during pregnancy.
Hematological toxicity is uncommon. Leukopenia, thrombocytopenia
and hemolytic anemia have been reported.
Altered coagulation may also occur with high intravenous
dosage. Phagocyte function may be impaired as a result of the
increased excretion of vitamin C.
Neurological toxicity is uncommon but includes benign
intracranial hypertension . A transient myopathy
has complicated long-term oral use for the treatment
of acne, while intravenous administration has caused
increased muscle weakness in those with myasthenia gravis
and has also potentiated curare-induced neuromuscular
blockade.
Metabolic effects include: precipitation of lactic acidosis
in diabetic patients receiving phenformin; a reduction
in vitamins B12, B6 and pantothenic acid with long-term
therapy; interference with laboratory tests of urinary catecholamines
and urinary tests for glucose (Clinitest and
Benedict’s); and elevation of serum lithium concentrations.
In addition, warfarin is potentiated and failure of oral
Contraception occurs. | | Synthesis | Tetracycline, 4-dimethylamino-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a�pentahydroxy-6-methyl-1,11-dioxo-2-naphthacencarboxamide (32.3.3), is synthesized by
reducing chlorotetracycline with hydrogen using a palladium on carbon catalyst. However, it
can be synthesized microbiologically using the actinomycete Streptomyces viridifaciens, as
well as a certain mutant S. aureofaciens. 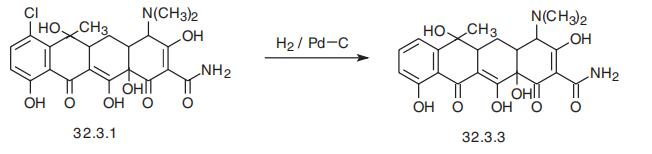
| | Potential Exposure | Tetracycline is an antibiotic medicine used as capsules, tablets, or intravenous injections against certain infections in humans and animals. | | Veterinary Drugs and Treatments | The tetracyclines are most useful in cats for the treatment of
Chlamydial and Mycoplasma conjunctivitis as well as nonspecific
or symptomatic therapy for undiagnosed (causative organism
not determined) conjunctivitis in cats. While its use in dogs and
horses is questionable, it may be useful in goats for Chlamydial/
Mycoplasma keratoconjunctivitis. At the time of publication, there
are no commercially available ophthalmic dosage forms of tetracycline.
There are, however, Veterinary-Labeled forms of oxytetracycline
and Polymyxin B ophthalmic ointments (Terramycin?). It is
again, important to note, that severe anaphylaxis, sometimes fatal,
has been associated with topical Polymyxin and neomycin in cats
and caution is recommended when using this product in cats. | | Drug interactions | Potentially hazardous interactions with other drugs
Anticoagulants: possibly enhance anticoagulant effect of coumarins and phenindione.
Oestrogens: possibly reduce contraceptive effects of oestrogens (risk probably small).
Retinoids: possible increased risk of benign intracranial hypertension with retinoids - avoid concomitant use. | | Metabolism | Tetracycline is excreted in the urine and in the faeces. Renal clearance is by glomerular filtration. Up to 60
% of an intravenous dose of tetracycline, and up to 55
% of an oral dose, is eliminated unchanged in the urine. The tetracyclines are excreted in the bile, where concentrations 5-25 times those in plasma can occur. There is some enterohepatic reabsorption and considerable quantities occur in the faeces after oral doses. | | Shipping | UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials. | | Purification Methods | Tetracycline crystallises from toluene or aqueous MeOH as the trihydrate. [Stephen et al. J Am Chem Soc 76 3568 1954, Beilstein 14 IV 2625.] | | Toxicity evaluation | Tetracycline is a potent inhibitor of bacterial protein
biosynthesis, with less activity on mammalian cells. It
binds to the 30S and 50S bacterial ribosomal subunits,
and it inhibits the binding of aminoacyl–tRNA and the
termination factors RF1 and RF2 to the A site of bacterial
ribosomes (21). Acute oral LD50 for mice >7 g/kg; for rats
>10 g/kg, acute intravenous 100~200 mg/kg. Tlm for
black bass: 250 ppm (24 h). | | Incompatibilities | Although no dangerous incompatibilities are reported, the potency of this medicine is reduced by heat, sunlight, and solutions with pH <2; and destroyed by caustic hydroxide solutions. | | Waste Disposal | It is inappropriate and possibly dangerous to the environment to dispose of expired or waste pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator. |
| | Tetracycline Preparation Products And Raw materials |
|