- 9-amino-CPT
-
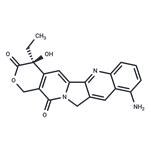
- $35.00 / 1mg
-
2024-11-19
- CAS:91421-43-1
- Min. Order:
- Purity: 99.82%
- Supply Ability: 10g
- 9-Aminocamptothecin
-
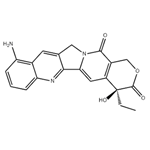
- $0.00 / 1KG
-
2022-10-14
- CAS:91421-43-1
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1Ton
- 9-Aminocamptothecin
-
- $0.00 / 1g
-
2020-07-27
- CAS:91421-43-1
- Min. Order: 1g
- Purity: 95%
- Supply Ability: 1kg/month
|
| | 9-Aminocamptothecin Basic information |
| Product Name: | 9-Aminocamptothecin | | Synonyms: | 4':6,7)indolizino(1,2-B)quinoline-3,14(4H,12H)-dione;4':6,7)indolizino(1,2-B)quinoline-3,14(4H,12H)-dione, 10-amino-4-ethyl-4-hydroxy-, (S)-;CAMPTOTHECIN, 9-AMINO-(RG);(S)-10-AMino-4-ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione;(S)-10-Amino-4-ethyl-4-hydroxy-1H-pyrano[3',4':6,7]-indolizino[1,2-b]quinoline-3,14(4H,12H)-di;CAMPTOTHECIN, 9-AMINO-;AMinocaMptothecin;9-AMINO-CAMPTOTHECIN | | CAS: | 91421-43-1 | | MF: | C20H17N3O4 | | MW: | 363.37 | | EINECS: | | | Product Categories: | Pharmaceutical Raw Materials;Alkaloids;Natural Anti-cancer Medical Materials and It's Derivatives | | Mol File: | 91421-43-1.mol | 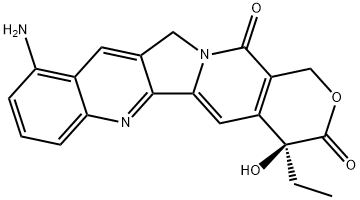 |
| | 9-Aminocamptothecin Chemical Properties |
| Melting point | 142.0-145.0 °C | | Boiling point | 819.6±65.0 °C(Predicted) | | density | 1.55±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C(protect from light) | | solubility | ≤1mg/ml in DMSO;1mg/ml in dimethyl formamide | | form | powder to crystal | | pka | 11.23±0.20(Predicted) | | color | Light yellow to Yellow to Orange | | Merck | 14,431 | | InChIKey | FUXVKZWTXQUGMW-FQEVSTJZSA-N | | CAS DataBase Reference | 91421-43-1(CAS DataBase Reference) |
| RTECS | UQ0490500 | | HS Code | 29349990 |
| | 9-Aminocamptothecin Usage And Synthesis |
| Uses | 9-Amino Camptothecin is a derivative of Camptothecin (C175150), as antitumor agent. | | Definition | ChEBI: 9-Aminocamptothecin is a pyranoindolizinoquinoline. | | Biological Activity | 9-amino camptothecin is a topoisomerase i inhibitor [1][2].dna topoisomerases relax dna torsional strain generated during replication, transcription, recombination, repair, and chromosome condensation. the relaxation of dna supercoiling by topoisomerase i is enabled by a mechanism of controlled rotation around a transient dna single-strand break. camptothecin (cpt) is isolated from the bark of the chinese tree camptotheca accuminata [3].9-amino camptothecin, a water-soluble camptothecin analogue, is a topoisomerase i inhibitor. in human ht-29 colon adenocarcinoma, 9-amino camptothecin (9-ac) exhibited cytotoxicity with ic50 value of 19 nm. 9-ac also induced dna damage in whole cells and nuclei at a concenstration of 85 nm and 21 nm, respectively [1].9-amino camptothecin had greater activity than camptothecin against human tumour xenografts, including lewis lung carcinoma and b16 melanoma. 9-ac had entered phase ii trials. in patients with advanced solid tumours, 9-amino camptothecin exhibited anti-tumor activity [1][2]. | | references | [1]. rothenberg, m.l. topoisomerase i inhibitors: review and update. annals of oncology 8(9), 837-855 (1997).
[2]. dancey j, eisenhauer ea. current perspectives on camptothecins in cancer treatment. br j cancer. 1996 aug;74(3):327-38.
[3]. drwal mn1, agama k, wakelin lp, et al. exploring dna topoisomerase i ligand space in search of novel anticancer agents. plos one. 2011;6(9):e25150. |
| | 9-Aminocamptothecin Preparation Products And Raw materials |
|