|
|
| | 4-Bromo-2-methylbenzoic acid Basic information |
| | 4-Bromo-2-methylbenzoic acid Chemical Properties |
| Melting point | 180-184 °C | | Boiling point | 310.1±30.0 °C(Predicted) | | density | 1.599±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | form | powder to crystal | | pka | 3.63±0.25(Predicted) | | color | White to Almost white | | Water Solubility | Insoluble in water. | | BRN | 2249816 | | InChI | InChI=1S/C8H7BrO2/c1-5-4-6(9)2-3-7(5)8(10)11/h2-4H,1H3,(H,10,11) | | InChIKey | RVCJOGNLYVNRDN-UHFFFAOYSA-N | | SMILES | C(O)(=O)C1=CC=C(Br)C=C1C | | CAS DataBase Reference | 68837-59-2(CAS DataBase Reference) |
| Hazard Codes | Xn,Xi | | Risk Statements | 22-36/37/38 | | Safety Statements | 26-36/37/39-36 | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 3 | | Hazard Note | Harmful | | HazardClass | IRRITANT | | PackingGroup | Ⅲ | | HS Code | 29163990 |
| | 4-Bromo-2-methylbenzoic acid Usage And Synthesis |
| Chemical Properties | White to brown crystal powde | | Uses | A building block which is used in preparation of anthranilic acids possessing antibacterial activity. 4-bromo-2-methylbenzoic acid could be used to synthesis 4-chloro-3'-(2-cyclopentyl-1-oxoisoindolin-5-yl)biphenyl-3-carboxylic acid through Suzuki couplings[1].
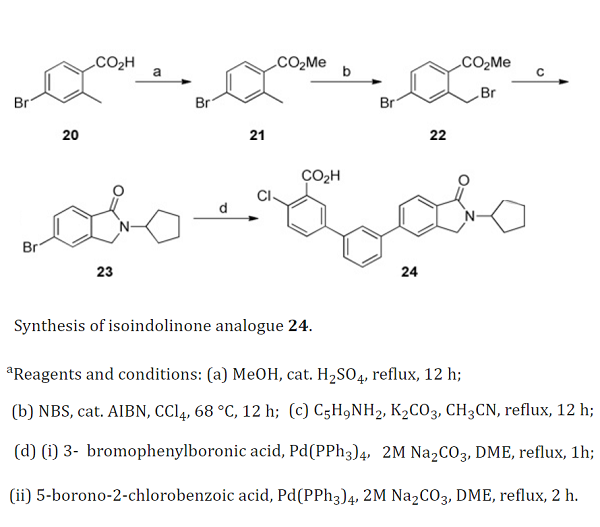 | | References | [1] Shyama Sidique. “Orally Active Metabotropic Glutamate Subtype 2 Receptor Positive Allosteric Modulators: Structure–Activity Relationships and Assessment in a Rat Model of Nicotine Dependence.” Journal of Medicinal Chemistry 55 22 (2012): 9434–9445.
|
| | 4-Bromo-2-methylbenzoic acid Preparation Products And Raw materials |
| Preparation Products | BENZOIC ACID,4-BROMO-2-METHYL-,METHYL ESTER-->5-Bromo-2-(bromomethyl)toluene, 4-Bromo-1-(bromomethyl)-2-methylbenzene-->4-acetyl-2-methylbenzoic acid-->4-bromo-2,6-dimethylbenzoic acid-->2,6-Piperidinedione, 3-[5-(aminomethyl)-1,3-dihydro-1-oxo-2H-isoindol-2-yl]--->METHYL 4-BROMO-2-BROMOMETHYL-BENZOATE-->4-BROMO-2-(CARBOXYMETHYL)BENZOIC ACID-->TERT-BUTYL 4-BROMO-2-METHYLBENZOATE-->4-bromo-N-methoxy-N,2-dimethylbenzamide-->2-METHYL-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)BENZOIC ACID |
|