Hydrocarbon halides
Hydrocarbon halide, or hydrocarbon for short, refers to the product produced during the process of hydrogen atoms replaced by by halogen (fluorine, chlorine, bromine, iodine) in the hydrocarbon molecules. According to the different hydrocarbon groups, it could be divided into categories like aliphatic halogenated hydrocarbons (including saturated and unsaturated halogenated hydrocarbons), aromatic halogenated hydrocarbons. According to the amount of halogen atoms contained in the molecule, it could be divided into categories like halogenated hydrocarbons, dihalogenated hydrocarbons and polyhalogenated hydrocarbons. Most of the halogenated hydrocarbons are liquid. The proportion of Iodinated hydrocarbons, brominated hydrocarbons and polyhalogenated hydrocarbons are all higher than 1. They are insoluble in water and could be dissolved in any proportion with hydrocarbons, they also could dissolve many other organics. The proportion of Hydrocarbon halide would decreases with the increase of the amount of carbon atoms. When there are the same hydrocarbon groups, the boiling point and the proportion of the halogenated hydrocarbons would increase in the order of chlorinated hydrocarbons, brominated hydrocarbons, and iodinated hydrocarbons. In the isomer, the more branches it gets, the lower boiling point it has. Most of these substances are toxic and can burn. And their combustion properties decrease with the increase of the amount of halogen atoms in the compound. When it comes to storage, fire hazard should be managed in A, B, C three categories according to fire risk indicators. When it catch fire, the gas should be treated by the C class of fire treatment, liquid by B class of fire treatment. Eenough attention should be payed to anti-virus.
The amount of naturally occurring halo hydrocarbons is very small, and the majority of halo hydrocarbons are synthesized by halogenation of alkanes, and compounded by unsaturated hydrocarbons. It also could be compounded during the reaction between alcohol with hydrohalic acid.
The amount of Halohydrocarbons are more isomers than corresponding hydrocarbons. Apart from the carbon isomerism, unsaturated bond position isomerization, cis-trans isomerism and optical isomerism, there are halogen atom isomerizations as well.. when the Halogenated hydrocarbon was named, the hydrocarbon plays a rule as the matrix while halogen atom plays as substituents. The position, number and name of halogen should be lined out before the name of the hydrocarbon. At room temperature, apart from methyl chloride, methyl bromide, ethyl chloride, vinyl chloride and others which are gas in room temperature, the halo hydrocarbon is liquid in general and the highly halogenated hydrocarbon is solid.
Halohydrocarbons is not dissolvable in water, but soluble in a variety of organic solvents, and can be dissolved in a variety of weak polarity and non-polar organic compounds. So it is a kind of commonly used solvent. The relative density of iodinated hydrocarbons, brominated hydrocarbons and polyhalogenated hydrocarbons are all higher than 1. The boiling point of halogenated hydrocarbons increases with the increasing atomic amount of halogen atoms. The boiling point of the same series of halogenated hydrocarbons increases with increase of the amount of carbon chain. The more branched the isomer, the lower the boiling point would it have (except from fluorinated alkanes).
The chemical property of halogenated hydrocarbons is much more active than paraffin, so halogenated hydrocarbons can produce a variety of chemical reactions, and turn them into various types of compounds. It has an important position in organic synthesis. Halo hydrocarbon has been applied widely in many trades, apart from being used as a solvent, it also could be used as refrigerant, extinguishing agent, anesthetics and preservatives.
Click on the specific product, view the latest prices of the products, information, serving information
| Structure |
Chemical Name |
CAS |
MF |
 |
1-Bromo-5-chloropentane |
54512-75-3 |
C5H10BrCl |
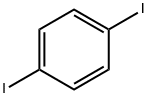 |
1,4-Diiodobenzene |
624-38-4 |
C6H4I2 |
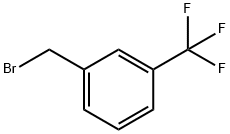 |
3-(Trifluoromethyl)benzyl bromide |
402-23-3 |
C8H6BrF3 |
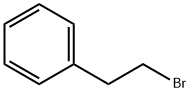 |
(2-Bromoethyl)benzene |
103-63-9 |
C8H9Br |
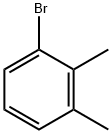 |
2,3-Dimethylbromobenzene |
576-23-8 |
C8H9Br |
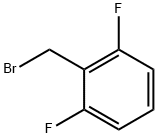 |
2,6-Difluorobenzyl bromide |
85118-00-9 |
C7H5BrF2 |
 |
Iodobenzene |
591-50-4 |
C6H5I |
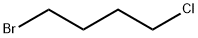 |
1-Bromo-4-chlorobutane |
6940-78-9 |
C4H8BrCl |
 |
1-Bromo-2-chloroethane |
107-04-0 |
C2H4BrCl |
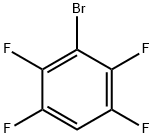 |
1-BROMO-2,3,5,6-TETRAFLUOROBENZENE |
1559-88-2 |
C6HBrF4 |
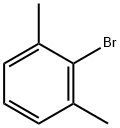 |
2-Bromo-m-xylene |
576-22-7 |
C8H9Br |
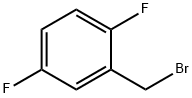 |
2,5-Difluorobenzyl bromide |
85117-99-3 |
C7H5BrF2 |
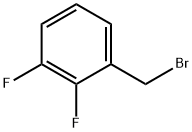 |
2,3-Difluorobenzyl bromide |
113211-94-2 |
C7H5BrF2 |
 |
1,5-Dibromopentane |
111-24-0 |
C5H10Br2 |
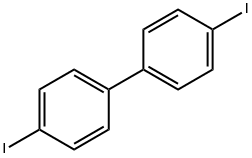 |
4,4'-Diiodobiphenyl |
3001-15-8 |
C12H8I2 |
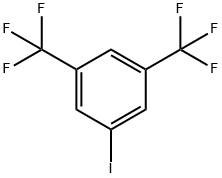 |
3,5-BIS(TRIFLUOROMETHYL)IODOBENZENE |
328-73-4 |
C8H3F6I |
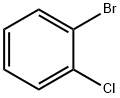 |
2-Bromochlorobenzene |
694-80-4 |
C6H4BrCl |
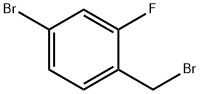 |
4-Bromo-2-fluorobenzyl bromide |
76283-09-5 |
C7H5Br2F |
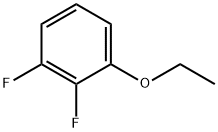 |
2,3-DIFLUOROETHOXYBENZENE |
121219-07-6 |
C8H8F2O |
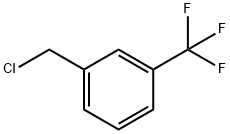 |
3-Chloromethyl-benzotrifluoride |
705-29-3 |
C8H6ClF3 |
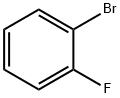 |
2-Bromofluorobenzene |
1072-85-1 |
C6H4BrF |
 |
1,3-Dichloropropane |
142-28-9 |
C3H6Cl2 |
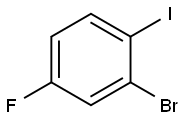 |
2-BROMO-4-FLUORO-1-IODOBENZENE |
202865-73-4 |
C6H3BrFI |
 |
1-Bromooctadecane |
112-89-0 |
C18H37Br |
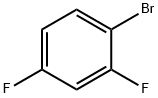 |
1-Bromo-2,4-difluorobenzene |
348-57-2 |
C6H3BrF2 |
 |
1-Bromohexadecane |
112-82-3 |
C16H33Br |
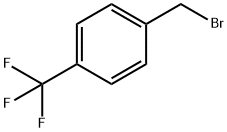 |
4-(TRIFLUOROMETHYL)BENZYL BROMIDE |
402-49-3 |
C8H6BrF3 |
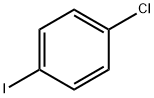 |
1-Chloro-4-iodobenzene |
637-87-6 |
C6H4ClI |
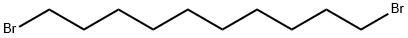 |
1,10-Dibromodecane |
4101-68-2 |
C10H20Br2 |
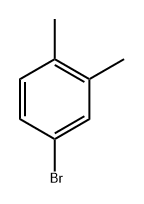 |
4-Bromo-o-xylene |
583-71-1 |
C8H9Br |
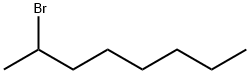 |
2-Bromooctane |
557-35-7 |
C8H17Br |
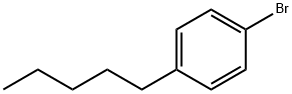 |
4-Pentylbromobenzene |
51554-95-1 |
C11H15Br |
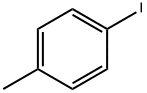 |
4-Iodotoluene |
624-31-7 |
C7H7I |
 |
1-Chlorotetradecane |
2425-54-9 |
C14H29Cl |
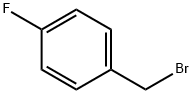 |
4-Fluorobenzyl bromide |
459-46-1 |
C7H6BrF |
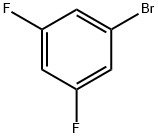 |
1-Bromo-3,5-difluorobenzene |
461-96-1 |
C6H3BrF2 |
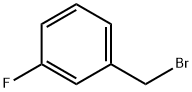 |
3-Fluorobenzyl bromide |
456-41-7 |
C7H6BrF |
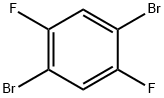 |
1,4-Dibromo-2,5-difluorobenzene |
327-51-5 |
C6H2Br2F2 |
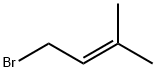 |
3,3-Dimethylallyl bromide |
870-63-3 |
C5H9Br |
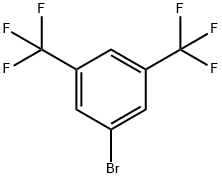 |
3,5-Bis(trifluoromethyl)bromobenzene |
328-70-1 |
C8H3BrF6 |
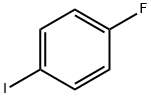 |
1-Fluoro-4-iodobenzene |
352-34-1 |
C6H4FI |
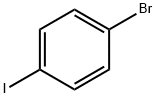 |
1-Bromo-4-iodobenzene |
589-87-7 |
C6H4BrI |
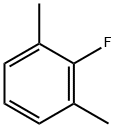 |
2,6-Dimethylfluorobenzene |
443-88-9 |
C8H9F |
 |
1-Bromohexane |
111-25-1 |
C6H13Br |
 |
1-Chlorooctadecane |
3386-33-2 |
C18H37Cl |
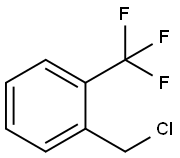 |
2-(Trifluoromethyl)benzyl chloride |
21742-00-7 |
C8H6ClF3 |
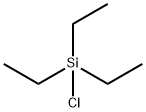 |
Chlorotriethylsilane |
994-30-9 |
C6H15ClSi |
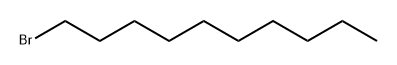 |
1-Bromodecane |
112-29-8 |
C10H21Br |
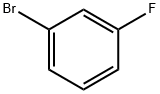 |
3-Bromofluorobenzene |
1073-06-9 |
C6H4BrF |
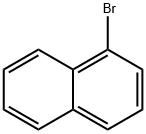 |
1-Bromonaphthalene |
90-11-9 |
C10H7Br |
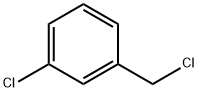 |
3-Chlorobenzyl chloride |
620-20-2 |
C7H6Cl2 |
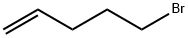 |
5-Bromo-1-pentene |
1119-51-3 |
C5H9Br |
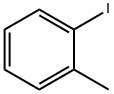 |
2-Iodotoluene |
615-37-2 |
C7H7I |
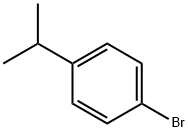 |
4-Bromocumene |
586-61-8 |
C9H11Br |
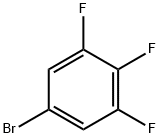 |
5-Bromo-1,2,3-trifluorobenzene |
138526-69-9 |
C6H2BrF3 |
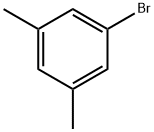 |
5-Bromo-m-xylene |
556-96-7 |
C8H9Br |
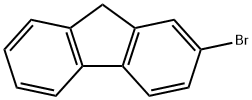 |
2-Bromofluorene |
1133-80-8 |
C13H9Br |
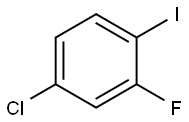 |
4-CHLORO-2-FLUOROIODOBENZENE |
6797-79-1 |
C6H3ClFI |
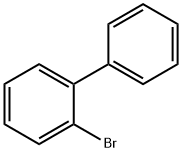 |
2-Bromobiphenyl |
2052-07-5 |
C12H9Br |
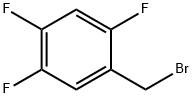 |
2,4,5-Trifluorobenzyl bromide |
157911-56-3 |
C7H4BrF3 |
 |
1,3-Dibromopropane |
109-64-8 |
C3H6Br2 |
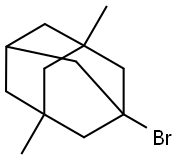 |
1-Bromo-3,5-dimethyladamantane |
941-37-7 |
C12H19Br |
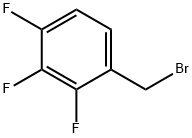 |
2,3,4-Trifluorobenzyl bromide |
157911-55-2 |
C7H4BrF3 |
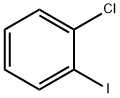 |
1-Chloro-2-iodobenzene |
615-41-8 |
C6H4ClI |
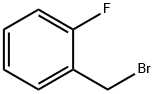 |
2-Fluorobenzyl bromide |
446-48-0 |
C7H6BrF |
 |
4-Bromo-1-butene |
5162-44-7 |
C4H7Br |
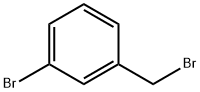 |
3-Bromobenzyl bromide |
823-78-9 |
C7H6Br2 |
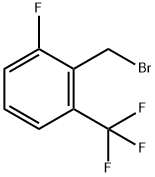 |
2-FLUORO-6-(TRIFLUOROMETHYL)BENZYL BROMIDE |
239087-08-2 |
C8H5BrF4 |
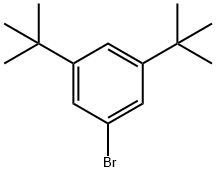 |
3,5-Di-tert-butylbromobenzene |
22385-77-9 |
C14H21Br |
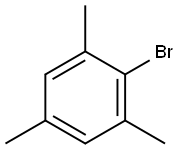 |
2,4,6-Trimethybromombenzene |
576-83-0 |
C9H11Br |
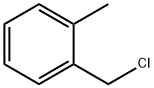 |
2-Methylbenzyl chloride |
552-45-4 |
C8H9Cl |
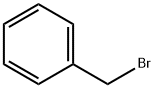 |
Benzyl bromide |
100-39-0 |
C7H7Br |
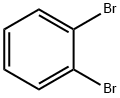 |
1,2-Dibromobenzene |
583-53-9 |
C6H4Br2 |
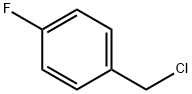 |
4-Fluorobenzyl chloride |
352-11-4 |
C7H6ClF |
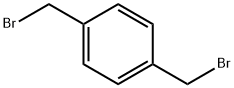 |
alpha,alpha'-Dibromo-p-xylene |
623-24-5 |
C8H8Br2 |
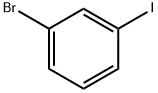 |
1-Bromo-3-iodobenzene |
591-18-4 |
C6H4BrI |
 |
1,4-Dibromobutane |
110-52-1 |
C4H8Br2 |
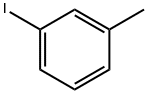 |
3-Iodotoluene |
625-95-6 |
C7H7I |
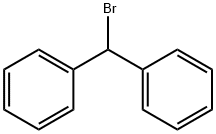 |
Bromodiphenylmethane |
776-74-9 |
C13H11Br |
 |
2,7-Dibromofluorene |
16433-88-8 |
C13H8Br2 |
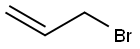 |
Allyl bromide |
106-95-6 |
C3H5Br |
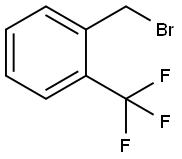 |
2-(Trifluoromethyl)benzyl bromide |
395-44-8 |
C8H6BrF3 |
 |
1-Bromotetradecane |
112-71-0 |
C14H29Br |
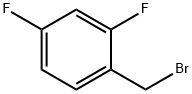 |
2,4-Difluorobenzyl bromide |
23915-07-3 |
C7H5BrF2 |
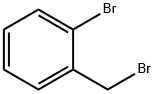 |
2-Bromobenzyl bromide |
3433-80-5 |
C7H6Br2 |
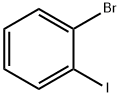 |
1-Bromo-2-iodobenzene |
583-55-1 |
C6H4BrI |
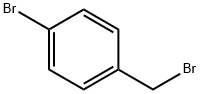 |
4-Bromobenzyl bromide |
589-15-1 |
C7H6Br2 |
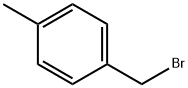 |
4-Methylbenzyl bromide |
104-81-4 |
C8H9Br |
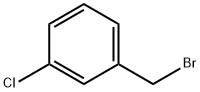 |
3-Chlorobenzyl bromide |
766-80-3 |
C7H6BrCl |
 |
1-Bromododecane |
143-15-7 |
C12H25Br |
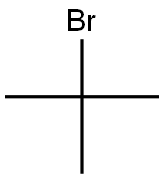 |
2-Bromo-2-methylpropane |
507-19-7 |
C4H9Br |
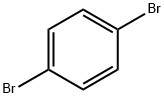 |
1,4-Dibromobenzene |
106-37-6 |
C6H4Br2 |
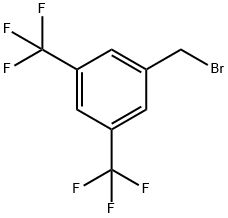 |
3,5-Bis(trifluoromethyl)benzyl bromide |
32247-96-4 |
C9H5BrF6 |
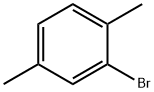 |
2,5-Dimethylbromobenzene |
553-94-6 |
C8H9Br |
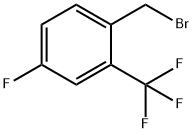 |
4-Fluoro-2-(trifluoromethyl)benzyl bromide |
206860-48-2 |
C8H5BrF4 |
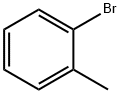 |
2-Bromotoluene |
95-46-5 |
C7H7Br |
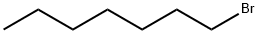 |
1-Bromoheptane |
629-04-9 |
C7H15Br |
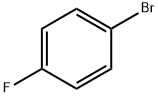 |
4-Bromofluorobenzene |
460-00-4 |
C6H4BrF |
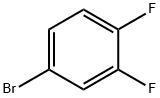 |
1-Bromo-3,4-difluorobenzene |
348-61-8 |
C6H3BrF2 |
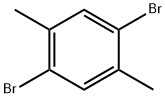 |
1,4-Dibromo-2,5-dimethylbenzene |
1074-24-4 |
C8H8Br2 |