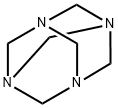
???
|
|
??? ??
- ???
- 280 °C (subl.) (lit.)
- ?? ?
- 246.7°C (rough estimate)
- ??
- 1.33
- ?? ??
- 4.9 (vs air)
- ???
- <0.01 mm Hg ( 20 °C)
- ???
- 1.4260 (estimate)
- ???
- 482 °F
- ?? ??
- Store below +30°C.
- ???
- H2O: 1 M at 20 °C, ??, ??
- ??? ??
- ??
- ??? ??
- ??? ??
- ?? ?? (pKa)
- 5.1(at 25℃)
- ??
- ???
- ??????(pH)
- 7-10 (100g/l, H2O, 20℃)
- ??
- ?? ??
- ???
- 895g/L(20℃)
- ??
- Hygroscopic
- ???
- 263-295 ºC
- Merck
- 14,5966
- BRN
- 2018
- ???
- ????. ??, ????? ???? ????.
- LogP
- -2.18--2 at 20℃
- CAS ??????
- 100-97-0(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | F,Xn,Xi | ||
|---|---|---|---|
| ?? ???? ?? | 11-42/43-43 | ||
| ????? | 16-22-24-37 | ||
| ????(UN No.) | UN 1328 4.1/PG 3 | ||
| WGK ?? | 1 | ||
| RTECS ?? | MN4725000 | ||
| F ?????? | 9-23 | ||
| ?? ?? ?? | 770 °F | ||
| TSCA | Yes | ||
| HS ?? | 2933 69 40 | ||
| ?? ?? | 4.1 | ||
| ???? | III | ||
| ?? ?? ??? | 100-97-0(Hazardous Substances Data) | ||
| ?? | LD50 orally in Rabbit: 9200 mg/kg | ||
| ???? ?? | KE-18615 | ||
| ???? ?? ??? | 60 |
??? C??? ??, ??, ??
??
Hexamethylenetetramine is a hardener in epoxy resins of the bisphenol A type and can also be used as an anticorrosive agent. It is a sensitizing agent in ceramics workers.??? ??
White or almost white, crystalline powder or colourless crystals.??
Hexamethylenetetramine is a versatile reagent in organic synthesis. It is used in the Duff reaction, the Sommelet reaction, and in the Delepine reaction.?? ??
Hexamethylenetetramine (also known as hexamine, hexa or HMT) is prepared from ammonia and formaldehyde: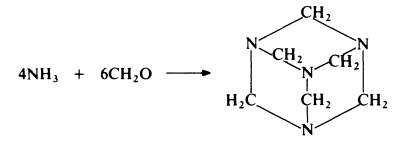
Ammonia is passed into formalin at 20-30??C, with agitation. The resulting solution is evaporated under reduced pressure until most of the water is removed and the hexamethylenetetramine crystallizes. Yields of about 95% on both ammonia and formaldehyde can be achieved. Hexamethylenetetramine is a colourless crystalline solid which, on heating, sublimes with decomposition.
??
ChEBI: A polycyclic cage that is adamantane in which the carbon atoms at positions 1, 3, 5 and 7 are replaced by nitrogen atoms.?? ??
Odorless white crystalline powder or colorless lustrous crystals. Sublimes in a vacuum at about 505° F with some decomposition. Solutions are strong bases (pH of 0.2 molar aqueous solution is 8.4).??? ?? ??
Highly flammable. Burns readily on contact with a flame with a smokeless flame. Finely powdered dust is significant dust explosion hazard. Water soluble.?? ???
Hexamethylenetetramine is hygroscopic. Hexamethylenetetramine is sensitive to exposure to heat. Hexamethylenetetramine is incompatible with oxidizing agents. Hexamethylenetetramine is also incompatible with acids. Hexamethylenetetramine reacts violently with sodium peroxide. Hexamethylenetetramine reacts explosively with 1-bromopentaborane(9) at temperatures above 194° F. The complex with iodine deflagrates at 280° F. The 1:1 addition complex with iodoform has exploded at 352° F. Hexamethylenetetramine is corrosive to some metals, such as aluminum and zinc . Special Hazards of Combustion Products: Formaldehyde gas and ammonia may be given off when hot [USCG, 1999].???
Skin irritant. Flammable, dangerous fire risk.????
Special Hazards of Combustion Products: Formaldehyde gas and ammonia may be given off when hot.?? ?? ??
Hexamethylenetetramine is used in the foundry, tire and rubber, and phenol formaldehyde resins industries and in other applications such as a hardener in epoxy resins Bisphenol A type and as an anticorrosive agent. It is an ammonia and formaldehyde releaser sometimes used in topical medicaments and cosmeticsClinical Use
A venerable drug used for the disinfection of acidic urine, methenamine is a low-molecular-weight polymer of ammonia and formaldehyde that reverts to its components under mildly acidic conditions. Formaldehyde is the active antimicrobial component. Methenamine is used for recurrent urinary tract infections. The drug is available in various dosage forms as well as various salts, including the hippurate and mandelate.Carcinogenicity
No significantly increased incidence of tumors was observed in rats or mice given HMTA for their lifetimes. Exposures in rats included 400 mg/day for 1 year, 10,000 ppm in drinking water for 2 years in each of three generations, 10,000 ppm in water for a lifetime (261), and up to 1000 ppm in the diet for 2 years. In mice, testing conditions included up to 10,000 ppm in drinking water for 60 weeks or 50,000 ppm for 30 weeks and a lifetime holding period, and up to 10,000 ppm in the diet for 2 years.Injection of 25–30 g subcutaneously per mouse led to an increase in subcutaneous sarcomas in two experiments (418, 419) but not in two other studies. The relevance of this methodology to the workplace condition is questionable.
Purification Methods
It is soluble in H2O (67%), CHCl3 (10%), EtOH (8%) and Et2O (0.3%), and a 0.2M solution has a pH of 8.4. Dissolve it in hot absolute EtOH (reflux, Norit), filter using a heated funnel, cool at room temperature first, then in ice. Wash the crystals with cold Et2O, dry them in air or under a vacuum. A further crop can be obtained by adding Et2O to the filtrate. It sublimes above 260o without melting. The picrate has m 179o(dec). [pK2 0 4.85: Reilley & Schmid Anal Chem 30 947 1958, pK2 0 6.30: Pummerer & Hofmann Chem Ber 56 1255 1923.] [Beilstein 26 I 306, 26 II 200, 26 III/IV 1680.]??? ?? ?? ? ???
???
?? ??
fluorescent whitening agent OM
4-???-2-????????
BENZP-??????-??-??
A-???-4-???????????
DL-THREO-2-ACETAMIDO-L-(4-?????)-L,3-?????
nitric acid pickling inhibitor LAN-5
???(4-)????
BIS(TRIMETHYLSILYL)PEROXIDE
4-???-3-????????
4-Cyanobenzaldehyde
3-Amino-2-azepanone
reversible temperature indicating coating (IV)
Nordazepam
???????
2-(4-???????)-1-????-5-???????
????? ????? ????
finishing agent KB for polyester viscose blend
4-tert-??????
2-(4-PENTYLBENZOYL)-1-BENZOFURAN-5-CARBALDEHYDE
2-(4-??????)-1-????-5-???????
???1,4-????-2,6-???-3,5-???????????
4-????????????
mnocyanide zine plating bright agent
2-(4-?????)-1-????-5-??????
4-N-???????
2,5-???????-3-?????????
??????????
triethylene tetraamine hexamethylene phosphoric acid
p-?? ???
5-??????
3,4-??????????
Amino moulding plastic
Nitrazepam
Scale inhibitor
??????
2-????-5-??-1,3-???????????
Estazolam
?????
2,5-DICHLOROTHIOPHENE-3-CARBOXYLIC ACID
N-[1-(??????)-2-(4-?????)-2-????]??????