ChemicalBook >?? ???? >2-???-6-????????
2-???-6-????????
|
|
2-???-6-???????? ??
- ???
- 129-131 °C (lit.)
- ?? ?
- 334.9±34.0 °C(Predicted)
- ??
- 1.2938 (rough estimate)
- ???
- 1.6800 (estimate)
- ?? ??
- Sealed in dry,Room Temperature
- ???
- ?????: ??? 5%, ??, ???? ??? ???
- ??? ??
- ??? ??
- ?? ?? (pKa)
- -1.49±0.10(Predicted)
- ??
- ????? ?? ?????
- InChI
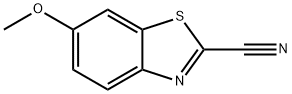
- InChI=1S/C9H6N2OS/c1-12-6-2-3-7-8(4-6)13-9(5-10)11-7/h2-4H,1H3
- InChIKey
- DEWDWBYQOFXKIH-UHFFFAOYSA-N
- SMILES
- S1C2=CC(OC)=CC=C2N=C1C#N
- CAS ??????
- 943-03-3(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xn,Xi | ||
|---|---|---|---|
| ?? ???? ?? | 20/21/22-36/37/38 | ||
| ????? | 26-36-36/37 | ||
| ????(UN No.) | 3439 | ||
| WGK ?? | 3 | ||
| HS ?? | 29341000 |
2-???-6-???????? C??? ??, ??, ??
??? ??
off-white to light yellow crystalline powder??
2-Cyano-6-methoxybenzothiazole has been used in the synthesis of:- firefly luciferin via condensation with cysteine
- luciferin β-glycosides, substrates for novel ultrasensitive enzyme assays
Synthesis
Typical routes to 2-cyano-6-methoxybenzothiazole include the classical Rosenmund-von Braun and Sandmeyer reactions. These methods proceed with low atom economy and require toxic reagents such as KCN, NaCN, Zn(CN)2, or TMSCN, which are also challenging to handle in a large-scale synthesis. Shahmoradi et al. introduced a Cu-catalyzed cyanation of 2-iodo-6-methoxybenzothiazole to synthesize 2-cyano-6-methoxybenzothiazole. K4[Fe(CN)6] was applied as a source of cyanide, and CuI in the presence of N, N N′, N′-tetramethylethylenediamine (TMEDA) was used as part of the catalyst system. 2-Amino-6-methoxybenzothiazole as a starting material was synthesized from p-anisidine as shown below and subsequently converted into 2-iodo-6-methoxybenzothiazole using a simple and efficient one-pot sequential diazotization-iodination method. The one-pot cyanation of 2-iodo-6-methoxybenzothiazole to 2-cyano-6-methoxybenzothiazole was achieved using 0.25 mmol of K4[Fe(CN)6], 0.25 mmol of CuI and 3 mmol of TMEDA in acetonitrile at 160°C. In addition, 1 mmol of mystril trimethyl bromide (MTMAB) was used as a phase transfer agent. The presence of a phase-transfer catalyst is essential for a successful cyanation reaction. Under these conditions, 2-cyano-6-methoxybenzothiazole was produced in a 90% yield[1].?? ??
[1] Ghasem Shahmoradi, S. Amani. “Synthesis, characterization and computational studies of 2-cyano-6-methoxybenzothiazole as a firefly-luciferin precursor.” Heterocyclic Communications 24 1 (2018): 255–258.2-???-6-???????? ?? ?? ? ???
???
??? ?I??
??????? ???
?????
??? ???
P-????
??????
??
??????(??)
??
??????
????(???)
???? ?????
??????
??????
?? ???
???? ??
Carbonocyanidothioicamide, N-(4-methoxyphenyl)-
2-Benzothiazolecarboxylicacid,6-methoxy-,methylester(7CI,8CI,9CI)
?? ??
2-???-6-???????? ?? ??
???( 223)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hongxiong | +undefined18915499068 |
Suzhouhongxiong@163.com | China | 120 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085; +8615531157085 |
abby@chuanghaibio.com | China | 8805 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3885 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12776 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2472 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3692 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29730 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-2158073036 |
info@dakenam.com | China | 19886 | 58 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 |
product@chemlin.com.cn | CHINA | 3009 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29856 | 58 |
2-???-6-???????? ?? ??:
?????? ????? ????? P-???? 2-????????? ?????????? ??????? 2-??? ??? ??? ??????????? ???????
2-METHYL-1,3-BENZOTHIAZOL-6-OL
6-METHOXY-2-METHYLBENZOTHIAZOLE
2-CYANO-6-HYDROXYBENZOTHIAZOLE
6-METHOXY-1,3-BENZOTHIAZOLE
2-Cyanothiazole
6-Benzothiazolol(7CI,8CI,9CI)
2-Methylthiazole
2-Cyano-6-methoxybenzothiazole