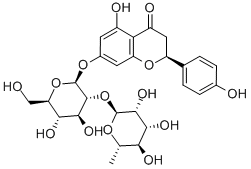
???
|
|
??? ??
- ???
- 166 °C
- ??
- -91 º (c=1, C2H5OH)
- ?? ?
- 559.35°C (rough estimate)
- ??
- 1.3285 (rough estimate)
- ???
- 2.25Pa at 20℃
- ???
- -84 ° (C=2, EtOH)
- ?? ??
- 2-8°C
- ???
- DMSO : 1 mg/mL (1.72 mM; Need ultrasonic)H2O : 1 mg/mL (1.72 mM; ultrasonic and warming and heat to 80°C)
- ??? ??
- ?? ??
- ?? ?? (pKa)
- 7.17±0.40(Predicted)
- ??
- ???
- optical activity
- [α]20/D 80±10°, c = 1% in ethanol
- ???
- ?, ???, ??? ? ??? ????? ?????.
- Merck
- 14,6425
- BRN
- 102012
- LogP
- -0.44 at 20℃
- CAS ??????
- 10236-47-2(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xn,Xi | ||
|---|---|---|---|
| ?? ???? ?? | 22-36/37/38 | ||
| ????? | 22-24/25-36-26 | ||
| WGK ?? | 3 | ||
| RTECS ?? | QN6340000 | ||
| F ?????? | 3 | ||
| TSCA | Yes | ||
| HS ?? | 29389090 |
??? C??? ??, ??, ??
????
? (1) ?? : ? ??? ?????? ?? ??? ?, ? ?? 2.0ppm ????? ??.
??(2) ? : ? ?? 5.0g? ??? ??????? ?? ?????????????? ?? ??? ?, ? ?? 5.0ppm ????? ??.
??(3) ???? : ? ??? 「????????」? ???? (5)? ?? ??? ?, ??? 50ppm ????? ??.
????
? (1) ? ?? 5mg? 50% ??? 10mL? ??? ??? ???????(1→500)? 1~2??? ???? ? ? ?? ??? ????.
??(2) ? ?? 5mg? ???????? 5mL? ??? ????? ? ? ?? ??~??? ????.
??(3) ? ?? 10mg? ? 500mL? ??? ?? ?? ?? 280~285nm?? ?????? ??.
???
? ? ??? 105℃? 3?? ???? ? ? 0.2g? ??? ?? 50% ???? ?? ? ??? 100mL? ??. ? ?? 0.45? ??? ???? ?, ? ? 1mL? ??? ??? ?? ??? 100mL? ? ?? ?????? ????. ?? ????? ?? 280nm??? ???? ???? ?? ???? ?? ??? ????.
????????????????????A : ????? ???
??
? ? ??? ??? ?(Citrus paradisi MACF.) ?? ??, ?? ?? ??? ? ?? ??? ??? ?? ???? ???? ?? ???? ???? ???? ? ??? ?????.
??
Naringin is also called naringoside, hesperidin, and isohesperidin. It is a pale yellow flavanone compound extracted from immature or nearly mature outer layer of C. paradisi Macfad which belongs to Citrus grandis (L.) Osbeck. It tastes bitter and is naturally existed in the skin and flesh of Rutaceae like grapefruit, mandarin orange, and orange. It is also one of the active ingredients in many traditional Chinese medi cines like Rhizoma Drynariae, Immature Bitter Orange, Fructus Aurantii, and Exocarpium Citri Grandis. The contents of naringin in different plants vary greatly with the category and origin, and the content of naringin is high in immature fruits . In terms of traditional Chinese medicine, the flavor of grapefruit is sweet and sour, and the nature is cold; the flavors of its peel are sweet, pungent, and bitter, and the nature of its peel is warm. Both the pulp and the peel of grapefruit have the bio logical function including reducing phlegm, helping digestion, relieving abdominal distention, and fast diaphragm. And they are mainly used for the treatment of cough with asthma, sense of suppression in the chest, coldness and pain in abdomen, dys peptic retention, and hernia.??? ??
beige to yellowish powder??? ??
Appearance: white to light yellow crystalline powder. Solubility: soluble in metha nol, ethanol, acetone, acetic acid, dilute alkali solution, and hot water and insoluble in nonpolar solvent like petroleum benzin, ether, benzene, and chloroform. Melting??
Naringin is mainly existed in the peel of grapefruit, lime, and their varieties; it has multiple biological activities and is widely applied in the fields of medicine, food, and cosmetics. In the 1930s, Harvey and Rygg obtained naringin through the method of isolation and extraction. They also established a colorimetric method for the determination of naringin, which laid the foundation for the following researches . Booth and other researchers conducted the systematic researches on the narin gin metabolites . In the 1960s, Hagen and other researchers established a fluores cence chromatography method for the determination naringin . In addition, the biological activity of naringin was evaluated. It was observed to improve ascites, experimental pulmonary edema, peritonitis, and oxygenation . At present, the extraction methods of naringin are hot water extraction, alkali extraction, and acid precipitation and organic solvent extraction. A series of pharmacological activity studies have been conducted and demonstrated its various biological activities .??
Naringoside is a metabolite of Naringin (N378980), a major flavonoid found in grapefruit juice. It has antioxidant, lipid lowering, and anticancer activities. It is also an inhibitor of cytochrome P450 enzymes, affecting drug metabolism and thus drug absorption in humans.??
ChEBI: A disaccharide derivative that is (S)-naringenin substituted by a 2-O-(alpha-L-rhamnopyranosyl)-beta-D-glucopyranosyl moiety at position 7 via a glycosi ic linkage.?? ??
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KGClinical Use
It was used for the treatment of bacterial infection, calm, and cancer preventionPurification Methods
This bitter principle from grape juice crystallises from water to give the hydrate with 6-8 H2O which when dried at 110o gives the dihydrate. Its solubility in H2O is 0.1% at 40o and 10% at 75o. The 2,4-dinitrophenylhydrazone crystallises from aqueous dioxane with m 246-247o [Douglass et al. J Am Chem Soc 73 4023 1951]. [Pulley & von Loesecke J Am Chem Soc 61 175 1939, Beilstein 18 III/IV 2637, 18 V 528.]??? ?? ?? ? ???
???
?? ??
??? ?? ??
???( 605)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Jiurui Biology Chemistry Co.,Ltd. | +86-0744-8561603 +8619974416182 |
sales11@jiuruibiochem.com | China | 26 | 58 |
| Changsha Staherb Natural Ingredients Co., Ltd. | +86-0731-84213302 +86-18374838656 |
info@staherb.cn | China | 1025 | 58 |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +8618829768577 |
gao490372054@gamil.com | China | 1249 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 3010 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12835 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5857 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18151 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 997 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 |
sarah@tnjone.com | China | 1143 | 58 |
??? ?? ??:
??? ??????? ??? P
Naringenin
Naringin dihydrochalcone
Hesperetin
Icariin
Naringenin 7-O-β-D-Glucuronide
(Mixture of Diastereomers)
(+/-)-Naringenin
NARINGENIN CHALCONE
Naringenin trimethyl ether
NARINGENIN
Naringin D4
4H-1-BENZOPYRAN-4-ONE, 2,3-DIHYDRO-7-METHOXY-
7-METHOXYFLAVANONE
Phloretin
NARIRUTIN
Neosperidin dihydrochalcone