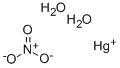???? ????? ???????
???? ????? ??????? ??
???
70 °C
??
4,79 g/cm3
?? ??
1.9 (vs air)
RTECS ??
OW8000000
???
slightly soluble in H2O
??? ??
?? ??
??
?? ??
??
?? ?? ??? ?? ??
???
?? ?? ?????.
??
Light Sensitive
Merck
14,5896
?? ??
ACGIH: TWA 0.025 mg/m3 (Skin)
CAS ??????
14836-60-3(CAS DataBase Reference)
??
?? ? ?? ??
?? ? ???? ?? (GHS)
??? ??
N-T+
?? ???? ??
50/53-33-26/27/28
?????
61-60-45-13
????(UN No.)
1627
TSCA
Yes
?? ??
6.1
????
II
HS ??
28521000
?????? ???
97-1-140
?? ? ????
????: ????; ???(??)????: ?? ?? ? ???? ?????? 1% ?? ??? ???. ?? ?? ????(Mercuric sulfide), ???? ????(Mercuric iodide), ???? ??(Mercuric oleate), ??? ?? ????(Amino mercury(II) chloride), ?? ????(Mercury(II) fulminate) ? ? ? ??? ??? ???? ??
????(GHS):
?? ?:
Danger
??·?? ??:
??
??·?? ??
?? ??
??
?? ?
?? ??
P- ??
H373 ??? ?? ?? ???? ??(??, ??? ?? ??? ?? ??? ??)? ??? ??? ? ??
?? ???? ?? - ?? ??
?? 2
??
P260, P314, P501
H410 ??? ??? ?? ????? ?? ???
?? ????? ?? - ??
?? 1
??
P273, P391, P501
??????:
P262 ?, ??, ??? ?? ??? ???.
P273 ???? ???? ???.
P280 ????/???/???/?????? ?????.
P314 ???? ??? ???? ??·??? ????.
NFPA 704
???? ????? ??????? C??? ??, ??, ??
??? ??
White to yellow crystalline powder
??
Mercury(I) nitrate dihydrate is used in the preparation of other mercury(I) compounds. It acts as a reducing agent. It is involved in the preparation of mercury(II) nitrate by treating with dilute nitric acid as well as to get mercury on exposure to light.
???? ????? ??????? ?? ?? ? ???
???
?? ??
???? ????? ??????? ?? ??
???( 71)?? ??
Canada 1
China 24
France 2
Germany 4
India 10
Japan 1
Mexico 1
Switzerland 1
United Kingdom 4
United States 23
Global 71
???? ????? ??????? ?? ??: