???? ?????? ???? C??? ??, ??, ??
??? ??
white or colourless crystals
??
It is used as a source of soluble arsenic.
??
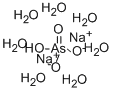
ChEBI: A hydrate that is the heptahydrate form of disodium hydrogen arsenate.
?? ??
Odorless white crystals. Becomes anhydrous at 212°F. Aqueous solution is alkaline to litmus.
??? ?? ??
Effloresces in warm air. Water soluble.
?? ???
SODIUM ARSENATE, HEPTAHYDRATE is incompatible with strong oxidizing agents and strong acids. Also incompatible with iron, aluminum and zinc in the presence of water. .
????
Arsenic is highly toxic. Exposures through ingestion and inhalation cause adverse health
effects that include, but are not limited to, irritation with itching, burning, and conjunctiva
damage, photophobia, corneal injury, dimness of vision, diplopia, lacrimation, cold
and clammy skin, low blood pressure, weakness, headache, cramps, infl ammation of the
mucous membranes with cough and foamy sputum, restlessness, dyspnea, cyanosis, pulmonary
edema, burning in the esophagus, vomiting, and bloody diarrhea, damage to the
liver and kidneys, and death from circulatory failure.
Repeated exposures to sodium arsenate heptahydrate cause bronzing of the skin, edema,
dermatitis, and lesions. Repeated or prolonged inhalation of dust may cause damage to the nasal septum. Chronic exposure from inhalation or ingestion may cause hair and weight
loss, a garlic odor to the breath and perspiration, excessive salivation and perspiration, central
nervous system damage, hepatitis, gastrointestinal disturbances, and cardiovascular
damage.
????
Literature sources indicate that SODIUM ARSENATE, HEPTAHYDRATE is nonflammable.
Safety Profile
Confirmed human
carcinogen. Poison by subcutaneous route.
An experimental teratogen. Other
experimental reproductive effects. Human
mutation data reported. See also ARSENIC
COMPOUNDS. When heated to
decomposition it emits toxic fumes of
arsenic.
??
Workers should keep containers of sodium arsenate heptahydrate tightly closed and under
lock and key in a cool, well-ventilated room/store area.
Purification Methods
Crystallise it from water (2mL/g).
?? ??
Occupational workers should avoid ingestion or breathing the dust of sodium arsenate
heptahydrate. On exposure to sodium arsenate heptahydrate, immediately fl ush eyes with
plenty of water. Occupational workers should wear suitable protective clothing, suitable
respiratory equipment, and work in proper ventilation. During use, workers should avoid
contact with the skin, eyes be away from incompatibles such as acids. For any kind of accidental
ingestion, workers should seek immediate medical advice.
Occupational workers and users should be aware that sodium arsenate heptahydrate is
very toxic and its improper use and handling is dangerous, causing fatal injury if swallowed
and/or inhaled. Sodium arsenate heptahydrate is a cancer hazard. It contains inorganic
arsenic that can cause cancer. The risk of cancer from sodium arsenate heptahydrate
depends on the duration and level of exposure. Because sodium arsenate heptahydrate
causes irritation to the skin, the eyes, and the respiratory tract, and causes liver and kidney
damage, workers should handle the chemical substance only with adequate ventilation,
respiratory equipment, and under qualifi ed supervision.
???? ?????? ???? ?? ?? ? ???
???
?? ??