??? CMC
|
|
??? CMC ??
- ???
- 274 °C (dec.)
- ??
- 1,6 g/cm3
- ?? ??
- room temp
- ???
- H2O: 20 mg/mL, ???
- ??? ??
- ??? ??
- ?? ?? (pKa)
- 4.30(at 25℃)
- ??
- ???? ?? ???
- ??????(pH)
- pH (10g/l, 25℃) 6.0~8.0
- ??
- ?? ??
- pH ??
- 6.5 - 8.5
- ???
- ??
- Merck
- 14,1829
- ???
- ????. ?? ???? ???? ????.
??? CMC C??? ??, ??, ??
????
? (1) ?? : ? ?? 0.5g? ??? ? 50mL? ???? ??? ??? 60~70℃?? ?? ????? 20?? ??? ??, ?? ? ???? ?????? ?? pH???? ?? ??? ?, pH? 6.0~8.5??? ??.
??(2) ??? : ? ?? 0.1g? ? 20mL ? ????? 0.5mL? ??? ?? ??? 30?? ???? ?? ?? ?? ??? 100mL? ?? ??? ??? ???? ?????. ?? 25mL? ?? ?? ?? ?????? ?? ??????? ?? ??? ?, ? ?? 0.01N ?? 0.45mL? ???? ? ????? ??.
??(3) ??? : ???? (2)?? ?? ?? 20mL? ?? ?? ?? ?????? ?? ??????? ?? ??? ?, ? ?? 0.01N ?? 0.4mL? ???? ? ????? ??.
??(4) ?? : ? ??? ?????? ?? ??? ?, ? ?? 4.0ppm ????? ??.
??(5) ? : ? ?? 5.0g? ??? ??????? ?? ?????????????? ?? ??? ?, ? ?? 2.0ppm ????? ??.
??(6) ??? : ? ?? 5.0g? ??? ??????? ?? ?????????????? ?? ??? ?, ? ?? 1.0ppm ????? ??.
??(7) ?? : ? ??? ?????? ?? ??? ?, ? ?? 1.0ppm ????? ??.
????
? (1) ? ?? 1g? ? 100mL? ??? ? ?? ??? ??? ??? ? ??? ???? ?? ?????? ?? ??? ??? ??.
????① ????? ?? ??? 5?? ???? ? ? 1??? ????????? 0.5mL? ??? ?? ??? 10?? ???? ???? ????.
????② ???? 5mL? ??? 10mL? ??? ? ??? ??? ??? ???? ??? ???.
????③ ???? 5mL? ?????(1→20) 5mL? ??? ??? ??? ?? ??? ??? ??? ???.
??(2) ? ?? 1g? 550~600℃?? 3?? ???? ?? ???? ????? ? ????? ??? ????.
??? ??
Carboxymethylcellulose sodium occurs as a white to almost white, odorless, tasteless, granular powder. It is hygroscopic after drying.??
In drilling muds, in detergents as a soil-suspending agent, in resin emulsion paints, adhesives, printing inks, textile sizes, as protective colloid in general. As stabilizer in foods. Pharmaceutic aid (suspending agent; tablet excipient; viscosity-increasing agent).??
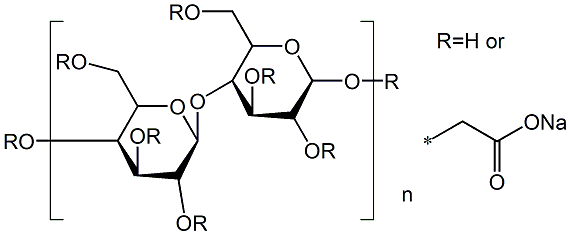
A semisynthetic, water-soluble polymer in which CH 2 COOH groups are substituted on the glucose units of the cellulose chain through an ether link- age. Mw ranges from 21,000 to 500,000. Since the reaction occurs in an alkaline medium, the prod- uct is the sodium salt of the carboxylic acid R-O- CH 2 COONa.?? ??
Alkali cellulose is prepared by steeping cellulose obtained from wood pulp or cotton fibers in sodium hydroxide solution. The alkaline cellulose is then reacted with sodium monochloroacetate to produce carboxymethylcellulose sodium. Sodium chloride and sodium glycolate are obtained as by-products of this etherification.?? ??
Sodium carboxymethyl cellulose (CMC) belongs to the class of anionic linear structured cellulose. Its components consist of polysaccharide composed of fibrous tissues of plants. It is a water soluble polymer which can be used as a polyelectrolyte cellulose derivative.Pharmaceutical Applications
Sodium carboxymethyl cellulose (NaCMC) is the sodium salt of carboxymethyl cellulose, an anionic derivative.It is widely used in oral and topical pharmaceutical formulations, primarily for its viscosity-increasing properties. Viscous aqueous solutions are used to suspend powders intended for either topical application or oral and parenteral administration. Carboxymethylcellulose sodium may also be used as a tablet binder and disintegrant, and to stabilize emulsions.Higher concentrations, usually 3–6%, of the medium-viscosity grade are used to produce gels that can be used as the base for applications and pastes; glycols are often included in such gels to prevent them drying out. Carboxymethylcellulose sodium is also used in self-adhesive ostomy, wound care, and dermatological patches as a muco-adhesive and to absorb wound exudate or transepidermal water and sweat. This muco-adhesive property is used in products designed to prevent post-surgical tissue adhesions; and to localize and modify the release kinetics of active ingredients applied to mucous membranes; and for bone repair. Encapsulation with carboxymethylcellulose sodium can affect drug protection and delivery. There have also been reports of its use as a cyto-protective agent.
Carboxymethylcellulose sodium is also used in cosmetics, toiletries, surgical prosthetics, and incontinence, personal hygiene, and food products.
Safety Profile
Mildly toxic by ingestion. Experimental reproductive effects. Questionable carcinogen with experimental neoplastigenic data. It migrates to food from packagmg materials. When heated to decomposition it emits toxic fumes of NazO. See also POLYMERS, SOLUBLE.Safety
Carboxymethylcellulose sodium is used in oral, topical, and some parenteral formulations. It is also widely used in cosmetics, toiletries, and food products, and is generally regarded as a nontoxic and nonirritant material. However, oral consumption of large amounts of carboxymethylcellulose sodium can have a laxative effect; therapeutically, 4–10 g in daily divided doses of the medium- and high-viscosity grades of carboxymethylcellulose sodium have been used as bulk laxatives.The WHO has not specified an acceptable daily intake for carboxymethylcellulose sodium as a food additive since the levels necessary to achieve a desired effect were not considered to be a hazard to health. However, in animal studies, subcutaneous administration of carboxymethylcellulose sodium has been found to cause inflammation, and in some cases of repeated injection fibrosarcomas have been found at the site of injection.
Hypersensitivity and anaphylactic reactions have occurred in cattle and horses, which have been attributed to carboxymethylcellulose sodium in parenteral formulations such as vaccines and penicillins.
LD50 (guinea pig, oral): 16 g/kg
LD50 (rat, oral): 27 g/kg
??
Carboxymethylcellulose sodium is a stable, though hygroscopic material. Under high-humidity conditions, carboxymethylcellulose sodium can absorb a large quantity (>50%) of water. In tablets, this has been associated with a decrease in tablet hardness and an increase in disintegration time.Aqueous solutions are stable at pH 2–10; precipitation can occur below pH 2, and solution viscosity decreases rapidly above pH 10. Generally, solutions exhibit maximum viscosity and stability at pH 7–9.
Carboxymethylcellulose sodium may be sterilized in the dry state by maintaining it at a temperature of 1608℃ for 1 hour. However, this process results in a significant decrease in viscosity and some deterioration in the properties of solutions prepared from the sterilized material.
Aqueous solutions may similarly be sterilized by heating, although this also results in some reduction in viscosity. After autoclaving, viscosity is reduced by about 25%, but this reduction is less marked than for solutions prepared from material sterilized in the dry state. The extent of the reduction is dependent on the molecular weight and degree of substitution; higher molecular weight grades generally undergo a greater percentage reduction in viscosity. Sterilization of solutions by gamma irradiation also results in a reduction in viscosity.
Aqueous solutions stored for prolonged periods should contain an antimicrobial preservative.
The bulk material should be stored in a well-closed container in a cool, dry place.
Purification Methods
Dialyse it for 48hours against distilled water and freeze-dry if a solid is required.? ???
Carboxymethylcellulose sodium is incompatible with strongly acidic solutions and with the soluble salts of iron and some other metals, such as aluminum, mercury, and zinc. It is also incompatible with xanthan gum. Precipitation may occur at pH < 2, and also when it is mixed with ethanol (95%).Carboxymethylcellulose sodium forms complex coacervates with gelatin and pectin. It also forms a complex with collagen and is capable of precipitating certain positively charged proteins.
Regulatory Status
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (dental preparations; intraarticular, intrabursal, intradermal, intralesional, and intrasynovial injections; oral drops, solutions, suspensions, syrups and tablets; topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.??? CMC ?? ?? ? ???
???
?? ??
??? CMC ?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Changshu Wealthy Science and Technology Co.,Ltd, | +8613706216163 |
baizhou@weiyichem.com | China | 1 | 58 |
| ZhenYiBio Technology Inc | +8615309206328 |
alexxue@zhenyibio.com | China | 296 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 |
admin@hbsaisier.cn | China | 1015 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 990 | 58 |
| Wuxi High Mountain Hi-tech Development Co.,Ltd. | +86-86-0510-85881806 +8613357920996 |
wuxihighmountain@gmail.com | China | 30 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12835 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5889 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18151 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 3010 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |