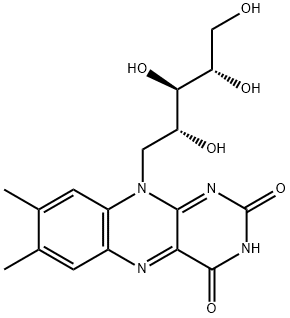
?????
|
|
????? ??
- ???
- 290 °C (dec.)(lit.)
- ??
- -135 º (c=5, 0.05 M NaOH)
- ?? ?
- 504.93°C (rough estimate)
- ??
- 1.2112 (rough estimate)
- ???
- -135 ° (C=0.5, JP Method)
- ???
- 9℃
- ?? ??
- 2-8°C
- ???
- ??? ?? ?? ??, ????? ?? ?? ????(96%). ?? ???? ?? ?? ?? ???? ??? ??? ?????. ???(5.9)? ?????.
- ??? ??
- ??
- ?? ?? (pKa)
- 1.7(at 25℃)
- ??
- ????? ?????
- ??
- ??? ??
- pH ??
- 6
- ??????(pH)
- 5.5-7.2 (0.07g/l, H2O, 20°C)
- ???
- 0.07g/L(20℃)
- ??
- Light Sensitive
- Merck
- 14,8200
- BRN
- 97825
- BCS Class
- 1
- ???
- ?????? ?? ?????. ?? ???, ???, ??, ??, ???? ???? ????. ??? ??? ? ????.
- InChIKey
- AUNGANRZJHBGPY-SCRDCRAPSA-N
- LogP
- -2.009 (est)
- CAS ??????
- 83-88-5(CAS DataBase Reference)
- NIST
- Riboflavine(83-88-5)
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | F,T | ||
|---|---|---|---|
| ?? ???? ?? | 11-23/24/25-39/23/24/25 | ||
| ????? | 24/25-45-36/37-16 | ||
| ????(UN No.) | UN1230 - class 3 - PG 2 - Methanol, solution | ||
| WGK ?? | 1 | ||
| RTECS ?? | VJ1400000 | ||
| F ?????? | 8-10-21 | ||
| TSCA | Yes | ||
| HS ?? | 29362300 | ||
| ?? ?? ??? | 83-88-5(Hazardous Substances Data) | ||
| ?? | LD50 in rats (g/kg): >10 orally, 5.0 s.c., 0.56 i.p. (Unna, Greslin) | ||
| ???? ?? | KE-11845 |
????? C??? ??, ??, ??
??
?????, ??? ??? B2?? ?? G? ??? ?? ?????, ??? ?? ??? ?? ?? ??? ?? ?? ??? ?? ?? ??? ??? ?? ??????. ??? B2? ??? ??? ??? ?? ?????. ??? ??? ?? ??? ??, ??? ??, ???, ???? ? ???? ?? ??? ?? ?? ??? ???.??
????? (??? B2)? ? ?? ?? ? ??, ???, ??? ??? ???, ?? ?? ?? ???? ???? ??? ?? ???? ?? ???? ??????.??? carbonhydrate, ??? ? ??? ?? ???, ????, ????, ?? ???? ??? ??, ??? ??? ?? ?????. ?? ?????, ??? perleche, ??, ophthalmitis, ???, seborrhea, ??? ??? ?? ?? ??? ?? ?????, ??? ?? ????, ?? ??? ?? ???? ?? ???? ??? ??? ??? ?????.
????
? (1) ???? : ? ??? ???? 105℃?? 2?? ??? ?? ? 50mg? ??? ?? 0.1N ???????? 2mL? ??? ??? ?? ??? ?? ? 5mL? ?? ?? ?? ??? ??? ???? ????? ???? ?? ??? 2mL? ??? ?? ??? ?? ?? ??? 10mL? ?? 30? ??? ? ?? ???? ??? ?, 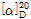 =-120~-140°??? ??.
=-120~-140°??? ??.
??(2) ????? : ? ?? 25mg? ??? ???? ?? ????? 10mL? ??? 5?? ??? ?? ?? ??? ?, ? ?? 0.1N ???????? 3mL? ?? ??? 1,000mL? ? ?? ??? ????? ?? ??.
??(3) ?? : ? ??? ?????? ?? ??? ?, ? ?? 4.0ppm ????? ??.
??(4) ? : ? ?? 5.0g? ??? ??????? ?? ?????????????? ?? ??? ?, ? ?? 2.0ppm ????? ??.
??(5) ??? : ? ?? 5.0g? ??? ??????? ?? ?????????????? ?? ??? ?, ? ?? 1.0ppm ????? ??.
??(6) ?? : ? ??? ?????? ?? ??? ?, ? ?? 1.0ppm ????? ??.
??(7) ????????1??? : ????? ? ????????1?????? ? ?, ? ?? ?????? 0.01% ????? ??.
????
? ? ?? 1mg? ? 100mL? ?? ?? ?? ?????? ?? ??? ? ??? ??? ??? ? ??? ???? ?? ????????? ??? ????.
???
? ? ??? ??? ?? ? 0.015g? ??? ?? ???(1→400) 800mL? ??? 60~70℃? ???? ??? ?? ?? ?? ??? 1,000mL? ?? ?? ?????? ??. ?? ???B2???? ???? ??? ??? ?? ???? ????? ??? ?? ??? ?? ?? 445nm?? ????? ??? AT ? ????? ??? AS? ??? ?? ?? ?? ? ?? ?????????? 0.02g? ??? ??? ?? ????? ? ?? ??? AT'' ? As''? ???? ?? ???? ?? ???B2(C17H20N4O6)? ??? ???. ??, ?? ??? ????? ??? ????? ????? ??.
?????
? ? ??? ?????? 0.3% ????? ??.
??
A water-soluble B fraction was found in the 1920s to contain a yellow, fluorescent growth factor called riboflavin in England and vitamin G in the United States. In the early 1930s, several groups found the coenzyme forms of riboflavin 50-phosphate (flavin mononucleotide) and the further conjugate with adenylic acid (flavin adenine dinucleotide).??? ??
VITAMIN B2 (Riboflavin). Some earlier designations for this substance included vitamin G, lactoflavin, hepatoflavin, ovoflavin, verdoflavin. The chemical name is 6,7-dimethyl-9-d-l’ribityl isolloxazine. Riboflavin is a complex pigment with a green fluorescence.??? ??
Riboflavin is moderately soluble in water (10–13 mg/dl) and ethanol but insoluble in ether, chloroform, and acetone. It is soluble but unstable under alkaline conditions.The catalytic functions of riboflavin are carried out primarily at positions N-1, N-5, and C-4 of the isoalloxazine nucleus. In addition, the methyl group at C-8 participates in covalent bonding with enzyme proteins. The flavin coenzymes are highly versatile redox cofactors because they can participate in either one- or two electron redox reactions
Riboflavin antagonists include analogs of the isoalloxazine ring (e.g., diethylri boflavin, dichlororiboflavin) and the ribityl side chain (e.g., d-araboflavin, d-galactoflavin, 7-ethylriboflavin).
??
riboflavin (Vitamin B2) is used in skin care preparations as an emollient. It can be found in sun care products as a suntan enhancer. Medicinally, it is used for the treatment of skin lesions.??
ChEBI: D-Ribitol in which the hydroxy group at position 5 is substituted by a 7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl moiety. It is a nutritional factor found in milk, eggs, malted barley, liver, kidney, heart, and leafy vege ables, but the richest natural source is yeast. The free form occurs only in the retina of the eye, in whey, and in urine; its principal forms in tissues and cells are as flavin mononucleotide and flavin-adenine dinucleotide.?? ??
The conflicting results were eventually found to be due,in part, to deficiencies in study animals not just of vitamin B2,but also vitamin B3 (niacin), the cause of human forms of pellagra,and/or vitamin B6 (pyridoxine), another cause of dermatitis.Likewise, treatments with vitamin B2 were inconsistentbecause the early sources of this vitamin contained otherB vitamins. Vitamin B2 was eventually isolated from eggwhites in 1933 and produced synthetically in 1935. Thename riboflavine was officially accepted in 1960; althoughthe term was in common use before then. In 1966, IUPACchanged it to riboflavin, which is in common use today.Riboflavin is synthesized by all green plants and by mostbacteria and fungi. Therefore, riboflavin is found, at least insmall amounts, in most foods. Foods that are naturally highin riboflavin include milk and other dairy products, meat,eggs, fatty fish, and dark green vegetables.Chemically, riboflavin is an N-glycoside of flavin, alsoknown as lumichrome, and the sugar, ribitol .Flavin is derived from the Latin word flavus for “yellow”because of the yellow color of its crystals and yellow fluorescenceunder UV light. Riboflavin is heat stable but easilydegraded by light. Its systematic names are 7,8-dimethyl-10-ribitylisoalloxazine and 7,8-dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)isoalloxazine.
Clinical Use
Severe riboflavin deficiency is known as ariboflavinosis, andtreatment or prevention of this condition is the only provenuse of riboflavin. Ariboflavinosis is most commonly associatedwith multiple vitamin deficiency as a result of alcoholismin developed countries. Because of the large numberof enzymes requiring riboflavin as a coenzyme, deficienciescan lead to a wide range of abnormalities. In adults seborrheicdermatitis, photophobia, peripheral neuropathy, anemia, andoropharyngeal changes including angular stomatitis, glossitis,and cheilosis, are often the first signs of riboflavin deficiency.In children, cessation of growth can also occur. As the deficiencyprogresses, more severe pathologies develop untildeath ensues. Riboflavin deficiency may also produce teratogeniceffects and alter iron handling leading to anemia.Safety Profile
Poison by intravenous route. Moderately toxic by intraperitoneal and subcutaneous routes. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.????
Physicochemical PropertiesRiboflavin has the appearance of a yellow to orange amorphous solid and imparts an orange color to the B vitamin tablets. Riboflavin has a melting point of 290°C, a density of 1.65, and a refractive index of 135°. The pKa is 9.888 and log P is 0.095. Riboflavin has solubility in water of 0.1 g l-1.
Exposure Routes and Pathways
The route of exposure is oral. Dietary sources of riboflavin and its coenzymes include broccoli, spinach, asparagus, enriched flour, yeast, eggs, milk, cheese, mackerel, trout, poultry, liver, and kidneys.
Toxicokinetics
Riboflavin, which is only moderately water soluble, is absorbed from the gastrointestinal tract but is limited to about 27 mg at any one time from an oral dose given to an adult. Hence, mega doses would not be expected to increase significantly the total amount absorbed. It is hepatically metabolized, protein bound, and widely distributed to tissue; however, little is stored in the liver, spleen, heart, and kidneys. Riboflavin is excreted renally as metabolites, which have been oxidatively cleaved in the ribityl side chain and converted to hydroxymethyls in the ring methyl functions. Riboflavin in excess of daily body needs is excreted unchanged in the urine. Riboflavin exhibits biphasic pharmacokinetics with initial and terminal half-lives of 1.4 and 14 h, respectively.
Purification Methods
It crystallises from H2O as a yellow-orange powder in three different forms with differing amounts of H2O. It melts if placed in an oil bath at 250o, but decomposes at 280o if heated at a rate of 5o/minute. It is also purified by crystallisation from 2M acetic acid, then extracted with CHCl3 to remove lumichrome impurity. [Smith & Metzler J Am Chem Soc 85 3285 1963.] Its solubility in H2O is 1g in 3-15L depending on the crystal structure. Its solubility in EtOH at 25o is 4.5mg in 100mL. Store it in the dark because it is decomposed to lumichrome by UV light. [Pearson The Vitamins vol V pp1-96 1967 and vol VII pp 1-96 1972, Gy.gy and Pearson eds, Academic Press, Beilstein 26 IV 2542.]????? ?? ?? ? ???
???
?? ??
????? ?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2930 | 58 |
| TAIZHOU YUXIN BIOTECHNOLOGY CO,.LTD | +86-576-88902229;+86-0576-88902229 +8613968687450 |
yuxin@yuxchem.com | China | 167 | 58 |
| XI AN SGONEK BIOLOGICAL TECHNOLOGY CO., LTD | 15202951039 |
jason@sgonekbio.com | China | 268 | 58 |
| Aurora Industry Co., Ltd. | +86-13591747876 +86-13591747876 |
alex1_auco@126.com | China | 277 | 58 |
| HUARONG(GUANGDONG) PHARMACEUTICAL CO.,LTD | +86-19925801629 +86-19925801629 |
eric@huarongpharma.com | China | 171 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8810 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 |
admin@china-yime.com | China | 563 | 58 |
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 18829239519 |
sales06@tgybio.com | China | 901 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5889 | 58 |
| Hebei Dangtong Import and export Co LTD | +86-13910575315 +86-13910575315 |
admin@hbdangtong.com | China | 1000 | 58 |