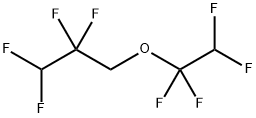
ChemicalBook >?? ???? >1,1,2,2-???????-3-(1,1,2,2-??????????)???
1,1,2,2-???????-3-(1,1,2,2-??????????)???
|
|
1,1,2,2-???????-3-(1,1,2,2-??????????)??? ??
- ?? ?
- 92 °C
- ??
- 1.533
- ???
- 1.29
- ???
- 27.5 °C
- ?? ??
- Sealed in dry,Room Temperature
- ??? ??
- ??? ??
- Specific Gravity
- 1.533
- ??
- ?? ?? ?? ??
- InChI
- InChI=1S/C5H4F8O/c6-2(7)4(10,11)1-14-5(12,13)3(8)9/h2-3H,1H2
- InChIKey
- HCBRSIIGBBDDCD-UHFFFAOYSA-N
- SMILES
- C(F)(F)C(F)(F)COC(F)(F)C(F)F
- CAS ??????
- 16627-68-2(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi | ||
|---|---|---|---|
| ?? ???? ?? | 36/37/38 | ||
| ????? | 26-36 | ||
| ????(UN No.) | 3271 | ||
| ?? ?? | IRRITANT | ||
| ???? | II | ||
| HS ?? | 29091990 | ||
| ???? ?? | 2009-1-593 | ||
| ?????? ??? | 2009-1-593 | ||
| ?? ? ???? | ????: ????; ???(??)????: 1,1,2,2-???????-3-(1,1,2,2-??????????)??? ? ?? 25% ?? ??? ??? |
1,1,2,2-???????-3-(1,1,2,2-??????????)??? C??? ??, ??, ??
??
1,1,2,2-Tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (HFE-458) is a hydrofluoroether. Hydrofluoroether is a new type of chlorofluorocarbons (CFCs) substitute, ODP is zero, GWP is low, and the atmospheric residence time is very short. It is considered as one of the ideal substitutes for CFCs.??
1,1,2,2-Tetrafluoroethyl-2,2,3,3-tetrafluoropropylether can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes.Synthesis
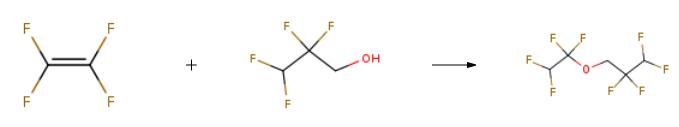
A system of a 6-L autoclave made of stainless steel was evacuated. The autoclave was charged with 401 g of potassium hydroxide (7.15 mol, 0.55 molar amount relative to 1 mol of a fluorine-containing alkyl alcohol), water (1604 mL), 2,2,3,3-tetrafluoro-1-propanol (boiling point of 109?? C., specific gravity of 1.4) represented by HCF2CF2CH2OH (1716 g, 13 mol) as a fluorine-containing alkyl alcohol. Then, the system was evacuated and replaced with nitrogen 20 times at room temperature. After evacuated, the system was filled with tetrafluoroethylene to 0.1 MPa, and the reaction system was heated to 85?? C. After the temperature in the system reached 85?? C., tetrafluoroethylene was added thereto little by little so that the reaction pressure was kept at 0.5 to 0.8 MPa. The temperature in the system was adjusted so as to be kept at 75 to 95?? C.Tetrafluoroethylene was added until the amount reached 0.5 molar relative to 1 mol of the fluorine-containing alkyl alcohol. The reaction was continuously carried out under stirring. When reduction in the pressure in the autoclave was stopped, the temperature in the autoclave was reduced to room temperature and unreacted tetrafluoroethylene was discharged, and the reaction was stopped. These steps took 5 hours to perform.The fluoroether of a lower phase of the production solution was HCF2CF2CH2OCF2CF2H (boiling point of 92?? C., specific gravity of 1.52). Table 1 shows the composition of the fluoroether production solution of a lower phase resulting from GC analysis.
1,1,2,2-???????-3-(1,1,2,2-??????????)??? ?? ?? ? ???
???
?? ??
1,1,2,2-???????-3-(1,1,2,2-??????????)??? ?? ??
???( 200)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
FandaChem@Gmail.com | China | 3315 | 55 |
| CD Chemical Group Limited | +8615986615575 |
info@codchem.com | China | 20342 | 58 |
| Wuhan Silworld Chemical Co.,Ltd | +86-027-85613400 +86-15827173649 |
info@silworldchemical.com | China | 178 | 58 |
| Shenzhen Huayi Brothers Technology Co.,Ltd | +8615914124590 |
ericzhang@szhybrother.com | China | 190 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5868 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2472 | 58 |
| Hebei Junhua Import and Export Co., LTD | +86-18503288844 +86-18503288844 |
junhua1@hb-junhua.com | China | 978 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-+86-371-66670886 |
info@dakenam.com | China | 19805 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29858 | 58 |
1,1,2,2-???????-3-(1,1,2,2-??????????)??? ?? ??:
N,N-???????????? ???? ?? ??? ????????? ?? ????? 1,1,2,2-???????-3-(1,1,2,2-??????????)???
ALLYL METHYL ETHER
(2-Methoxyethyl)benzene
1H,1H,5H-Perfluoropentyl-1,1,2,2-tetrafluoroethylether
PERFLUORO(1-ETHYL-3-PROPOXYCYCLOHEXANE)
1H,1H-PERFLUORO-3,6,9-TRIOXATRIDECAN-1-OL
PERFLUORO(1,3-DIPROPOXYCYCLOHEXANE)
PERFLUORO-3,6,9-TRIOXATRIDECANOIC ACID
PERFLUORO-3,6-DIOXADECANOIC ACID
BIS(4-CHLOROOCTAFLUOROBUTYL)ETHER
METHYL PERFLUORO-3,6,9-TRIOXATRIDECANOATE
METHYL PERFLUORO-3,6-DIOXADECANOATE
1-BROMOPERFLUORO-2,5-DIOXANONANE
1-BROMOPERFLUORO-2,5,8-TRIOXADODECANE