???? ??? (II)
|
|
???? ??? (II) ??
- ???
- 205 °C
- ???
- 0.002Pa at 25℃
- ?? ??
- Store below +30°C.
- ???
- ???? ??? ?? ??? ???? ?????.
- ??? ??
- ???? ??? ??-(??/????/???/??)
- ??
- ???
- ??????(pH)
- 2-3 (H2O, 20℃)(aqueous suspension)
- pH ??
- 2.0 - 3.0 at 20 °C
- ???
- ???
- ??
- Hygroscopic
- Hydrolytic Sensitivity
- 4: no reaction with water under neutral conditions
- ?? ??(λmax)
- 400nm(EtOH)(lit.)
- Merck
- 14,6991
- BRN
- 6086766
- InChIKey
- PCUVQHHZCJMCHO-UHFFFAOYSA-M
- LogP
- -0.17 at 20℃
- CAS ??????
- 3375-31-3(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi,Xn,C | ||
|---|---|---|---|
| ?? ???? ?? | 41-36/37/38-40-35 | ||
| ????? | 26-39-36/37/39-45-36 | ||
| ????(UN No.) | 3261 | ||
| WGK ?? | 2 | ||
| RTECS ?? | AJ1900000 | ||
| F ?????? | 10-23 | ||
| TSCA | Yes | ||
| HS ?? | 28439090 | ||
| ?? | LD50 orally in Rabbit: >= 5110 mg/kg | ||
| ?????? ??? | 2017-1-766(??????) | ||
| ?? ? ???? | ????: ????; ???(??)????: ???? ???(Ⅱ) ? ?? 1.0% ?? ??? ??? |
???? ??? (II) C??? ??, ??, ??
??? ??
Brown needles, soluble in benzene and toluene, insoluble in ether and alcohol, keep in dark and refrigerated.??
Palladium (II) Acetate Trimer is used in Suzuki-Miyaura cross-coupling reactions and a catalyst for intramolecular coupling. It also serves to catalyze the chemoselective reduction of nitroarenes.??
Palladium acetate is a chemical compound of palladium. It is used as a catalyst for many organic reactions and as a precursor to other palladium(II) compounds. Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is found as a free metal alloyed with gold and other platinum group metals and in the rare minerals cooperite and polarite. (Toxin and Toxin Target Database (T3DB))?? ??
The synthesis of palladium acetate was first reported by Wilkinson et al.,where glacial acetic acid containing palladium powder is heated at reflux with a minimal amount of nitric acid until the generation of brown NOx fumes ceases. Although this is a relatively old method, it is still the most commercially employed route for the synthesis of palladium acetate, considering the process economics. Palladium (II) Acetate can be synthesized in two steps from palladium sponge via the intermediate dinitrate by first combining it with hot glacial acetic acid and nitric acid:Pd + 2 HNO3 → Pd(NO3)2 + H2
Pd(NO3)2 + 2 CH3COOH → Pd(O2CCH3)2 + 2 HNO3
?? ??
- Efficient catalyst for the arylation of olefins (Heck reaction).
- Catalyst for cross-coupling reactions.
- Catalyst for C-H activation.
- Precatalyst for enantioselective decarboxylative protonation of allyl β-ketoesters.
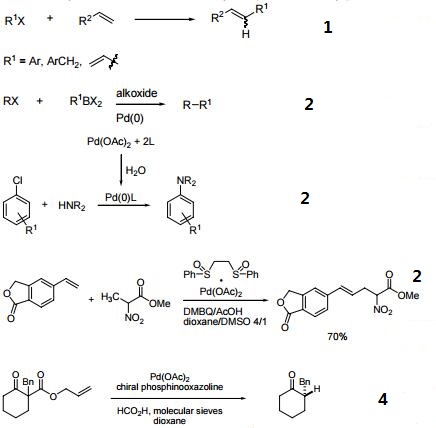
?? ??
Palladium(II) acetate is a homogenous oxidation catalyst. It participates in the activation of alkenic and aromatic compounds towards oxidative inter- and intramolecular nucleophilic reactions. Crystals of palladium(II) acetate have a trimeric structure, having symmetry D3h. Each of the palladium atoms in the crystals are joined to the other two by double acetate bridges. Microencapsulation of palladium(II) acetate in polyurea affords polyurea-encapsulated palladium(II) acetate. It is a versatile heterogeneous catalyst for various phosphine-free cross-coupling reactions. It participates as catalyst in the Heck coupling reaction of pthalides with different alkenes.Safety Profile
Moderately toxic by ingestion. When heated to decomposition it emits toxic vapors of palladium.Purification Methods
It recrystallises from CHCl3 as purple crystals. It can be washed with AcOH and H2O and dried in air. Large crystals are obtained by dissolving it in *C6H6, adding half its volume of AcOH and allowing it to evaporate slowly at room temperature. It forms green adducts with nitrogen donors, it dissolves in KI solution to form solid PdI2 and a red solution of PdI42-, but is insoluble in aqueous saturated NaCl, and NaOAc. It dissolves in HCl to form PdCl42-. It is soluble in CHCl3, CH2Cl2, Me2CO, MeCN, Et2O, but it is insoluble in H2O, and decomposes when warmed in alcohols in which it is also insoluble. [Morehouse et al. Chem Ind (London) 544 1964, Stephenson et al. J Chem Soc 3632 1965, Skapski & Smart J Chem Soc (D) 658 1970, Heck Acc Chem Res 12 146 1979.]???? ??? (II) ?? ?? ? ???
???
?? ??
3- ??? ? ??
2,3-????-1H-??-5-?????
3-??????
N,N'-Bis- (1-naphthalenyl)-N,N'-bis-phenyl-(1,1'-biphenyl)-4,4'-diamine
5-???-2,3-?????-1H-??
??(R)-2-?????-4-???????
(E)-METHYL 3-(4-BROMOPHENYL)ACRYLATE
(S)-(-)-7,7'-BIS[DI(3,5-DIMETHYLPHENYL)PHOSPHINO]-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE
5-?????-2-????????
C-(3,4-DIHYDRO-2H-BENZO[1,4]OXAZIN-3-YL)-????
(S)-7,7'-??[?(p-????)????]-1,1'-?????
4-(2-??-1-???????)????
1-???-5-???-2,3-?????-(1H)-??
ethyl 1H-indole-6-carboxylate
(R)-(+)-7,7'-BIS[DI(4-METHYLPHENYL)PHOSPHINO]-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE
2-MORPHOLINO-5-(TRIFLUOROMETHYL)??????
4-????? ??? ??? ????
(R)-(+)-7,7'-BIS(DIPHENYLPHOSPHINO)-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE
???????
?????-6-???????
5-?????-2-?????
5-??????
4-??????
INDOLINE-6-CARBOXYLIC ACID
3-??-1H-???
1-(4-Pyridyl)piperazine
??????
5-????
4,6-Diamino-2-pyrimidinol
(R)-7,7'-BIS(DIPHENYLPHOSPHINO)-1,1'-SPIROBIINDANE
???? ??? (II) ?? ??
???( 621)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3882 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8615713292910 |
Nancy@kangcang.com.cn | China | 341 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 |
admin@hbsaisier.cn | China | 1015 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 +86-18633929156 |
admin@hblongbang.com | China | 941 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 |
L18532138899@163.com | China | 939 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 |
info@spring-chem.com | China | 2068 | 57 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18751 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 |
FandaChem@Gmail.com | China | 9095 | 55 |