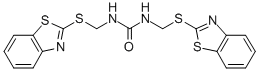
1,3-bis(2-benzothiazolylmercaptomethyl)urea C??? ??, ??, ??
??? ??
tan to yellow powder
?? ??
Buff to light tan or light yellow powder.
??? ?? ??
Insoluble in water.
?? ???
An organosulfide, amide. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
????
SYMPTOMS: Related compounds have been reported to cause central nervous system depression (e.g. drowsiness, decreased mentation).
????
Flash point data for 1,3-bis(2-benzothiazolylmercaptomethyl)urea are not available. 1,3-bis(2-benzothiazolylmercaptomethyl)urea is probably combustible.
1,3-bis(2-benzothiazolylmercaptomethyl)urea ?? ?? ? ???
???
?? ??