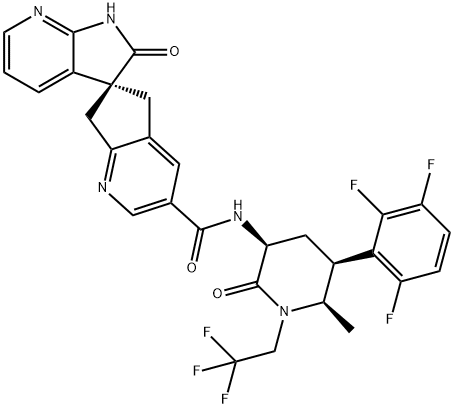
Atogepant (MK-8031)
- CAS No.
- 1374248-81-3
- Chemical Name:
- Atogepant (MK-8031)
- Synonyms
- MK-8031;AGN-241689);Atogepant API;MK8031. Atogepant;Atogepant Monohydrate;(3'S)-1',2',5,7-tetrahydro-N-[(3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)-3-piperidinyl]-2'-oxo-Spiro[6H-;(S)—N-((3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl)-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamid;(S)-N-((3S,5S,6R)-6-Methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl)-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamide;(S)—N-((3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl)-2’-oxo-1’,2’,5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3’-pyrrolo[2,3-b]pyridine]-3-carboxamide;(3'S)-N-[(3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl]-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamide
- CBNumber:
- CB83368673
- Molecular Formula:
- C29H23F6N5O3
- Molecular Weight:
- 603.52
- MDL Number:
- MOL File:
- 1374248-81-3.mol
| Boiling point | 693.9±55.0 °C(Predicted) |
|---|---|
| Density | 1.54±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| pka | 11.08±0.20(Predicted) |
| form | Solid |
| color | White to off-white |
| InChIKey | QIVUCLWGARAQIO-OLIXTKCUSA-N |
| SMILES | C12C[C@@]3(C(=O)NC4=NC=CC=C43)CC1=CC(C(N[C@H]1C[C@@H](C3=C(F)C=CC(F)=C3F)[C@@H](C)N(CC(F)(F)F)C1=O)=O)=CN=2 |
| FDA UNII | 7CRV8RR151 |
Atogepant (MK-8031) Chemical Properties,Uses,Production
Structure
Atogepant (MK-8031/AGN-241689) is a polycyclic molecule containing four chiral centers and three aromatic and aliphatic rings. An intriguing feature is the 2-azaspiro [4.4]nonan-1-one (spiroazaindane) motif in an S configuration, also present in earlier preclinical leads from Merck. The azospiro bispyridine unit is linked via an amide bond to a piperidine-2-one ring; the amide linkage is reversed to that seen in earlier leads to avoid the potential release of a toxic aniline. The piperidine-2-one ring is heavily functionalized with 6-(R)-methyl, 5-(R)-2,3,6-trifluorophenyl substituents. The 2,3,6 pattern of fluoro substituents provided a ~fourfold increase in atogepant affinity compared with ubrogepant, which contains an unsubstituted phenyl ring. The fluorine substitution pattern in atogepant may be responsible for alleviating the hepatotoxicity associated with some other gepants, such as telcagepant. Notably, the piperidinone is masked with a 2,2,2,-trifluoroethyl group, a substitution that provided improved telcagepant's potency, bioavailability, and half-life. Atogepant’s molecular weight (603 g/mol), the presence of five H-bond acceptors and two H-bond donors, and its large polar surface area (109.29 ?2) may limit penetration via passive diffusion through the blood-brain barrier.
Uses
Atogepant (MK-8031) is an orally active and selective calcitonin gene–related peptide receptor (CGRP) antagonist. Atogepant can be used for researching migraine.
Definition
ChEBI: Atogepant is a secondary carboxamide resulting from the formal condensation of the carboxy group of (3'S)-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxylic acid with the amino group of (3S,5S,6R)-3-amino-6-methyl-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-2-one. It is a selective oral, small-molecule antagonist of calcitonin gene-related peptide (CGRP) receptor that has been approved for the treatment of migraine. It has a role as a calcitonin gene-related peptide receptor antagonist. It is a secondary carboxamide, an azaspiro compound, an organic heterotetracyclic compound, a trifluorobenzene and a member of piperidones.
Mechanism of action
Atogepant (MK-8031) is an antagonist of the calcitonin gene-related peptide receptor. It competes with CGRP for occupancy at these receptors, preventing the actions of CGRP and its ability to induce and perpetuate migraine headache pain.
Side effects
The side effect profile of atogepant is similar to other gepants, most frequently including constipation, nausea, upper respiratory and urinary tract infection, and appearing similarly in long-term follow-up studies and clinical trials. Currently, there is no data on the safety of atogepant treatment during pregnancy or lactation. However, it can be assumed, based on animal data, that it is able to pass into breast milk.
Synthesis
The synthesis of atogepant was carried out via amide coupling of two advanced intermediate fragments. The specific route is as follows:
Step 1: The synthesized carboxylic acid fragment started from the biscarboxylic acid 10.1, which was converted to the bisester under acidic conditions and then brominated to give the bromopyridine 10.2 (Fig. 1). 10.3 was obtained by reduction of the two esters and subsequent mesylation. Treatment of the mesylate 10.3 with the pyridone 10.4 under basic conditions gave the racemic spirocyclic compound 10.5. Palladium-catalyzed carbonylation gave the methyl ester, which was purified by chiral supercritical fluid chromatography (SFC) to give the single enantiomer pyridine 10.6. Ester hydrolysis and deprotection of the SEM protecting group gave the desired carboxylic acid fragment 10.7.
Step 2: The amine fragment synthesis was performed by converting the carboxylic acid 10.11 to the corresponding Weinreb amide 10.12 (N-methoxy-N-methylamide) by generating the acid chloride using POCl3 (Fig. 2). The Weinreb amide reacted with the Grignard reagent methylmagnesium chloride to give the methyl ketone 10.13. Enolization of the ketone 10.13 was achieved using LiOt-Bu and ZnBr2. Subsequently, treatment with the racemic mesylate 10.14 gave the amino acid derivative 10.15. Enzymatic reductive amination of the ketone 10.15 and transaminase-induced cyclization gave the pyridone 10.16 with a >60:1 cis-trans ratio at C5:C6 and a 1:1 mixture of cis-trans isomers at C3. Epimerization of 10.16 with t-BuOK gave the pyridone 10.17. Selective N-alkylation with the triflate 10.18 gave the pyridone 10.19 with a 6.5:1 mixture of cis-trans isomers at the C3 position. The primary amine at the C3 position was obtained by acidic deprotection and then treated with amino acid 10.20 to generate salt 10.21. Finally, amine 10.21 was treated as a free base and reacted with carboxylic acid 10.7 via EDC coupling to afford Atogepant (10) in 95% yield.
in vivo
Atogepant (3, 10, 30 mg/kg; Oral gavage (p.o.); Once a day; 9 days) improvement is observed in abnormal facial pain induced by nitroglycerin in rats, and in capsaicin-induced dermal vasodilation in primates[2].
Atogepant concentrations and concentration ratios in plasma, cerebrospinal fluid and brain tissue (Sprague-Dawley rats)[2]
| Parameter | Plasma Cmax (ng/mL) | CSF Cmax (ng/mL) | Brain Cmax (ng/g) | CSF/plasma Cmax ratios (ng/ml) | Brain/plasma Cmax ratios (ng/ml) | Brain/CSF Cmax ratios (ng/ml) | Plasma AUC0-last (ng h/ml) | CSF AUC0-last (ng h/ml) | Brain AUC0-last (ng h/g) | CSF/plasma AUC0-last ratios (ng h/ml) | Brain/plasma AUC0-last ratios (ng h/ml) | Brain/CSF AUC0-last ratios (ng h/ml) |
| Atogepant 5 mg/kg | 180.7 | 0.9 | 2.4 | 0.005 | 0.0133 | 2.6667 | 1110.9 | 4.2 | 17.9 | 0.0038 | 0.0161 | 4.2619 |
| Atogepant 20 mg/kg | 853.7 | 3.7 | 17.3 | 0.0043 | 0.0203 | 4.6757 | 6511.7 | 20.0 | 102.3 | 0.0031 | 0.0157 | 5.1150 |
Atogepant (MK-8031) Preparation Products And Raw materials
Raw materials
Preparation Products
Atogepant (MK-8031) Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Jingkang en Biomedical Technology Co., Ltd | +8613720134139 | orders@jknbiochem.com | China | 5221 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659 +86-13153181156 | sales@sdperfect.com | China | 294 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | figo.gao@foxmail.com | China | 8497 | 58 |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | contact@trustwe.com | China | 5745 | 58 |
| TargetMol Chemicals Inc. | +17819995354 | marketing@targetmol.com | United States | 32286 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6312 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10473 | 58 |
| SyncoZymes (Shanghai) Co., Ltd., | +8613681683526 | lchen@syncozymes.com | China | 188 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38669 | 58 | |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8618501500038 | 3899766280@qq.com | China | 26362 | 58 |
Related articles
- How is Atogepant synthesised?
- Atogepant was synthesised from the late-stage amide coupling of two highly complex fragments (Carboxylic Acid Fragment and Spi....
- Jan 15,2024
View Lastest Price from Atogepant (MK-8031) manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-06-09 | Atogepant (MK-8031)
1374248-81-3
|
US $0.00-0.00 / KG | 1KG | 98.0% | 10000KGS | Shaanxi Dideu New Materials Co. Ltd | |
 |
2025-06-04 | Atogepant
1374248-81-3
|
US $158.00-347.00 / mg | 99.66% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Atogepant
1374248-81-3
|
US $0.00 / g | 1g | 98% HPLC | 1kg | shandong perfect biotechnology co.ltd |
-

- Atogepant (MK-8031)
1374248-81-3
- US $0.00-0.00 / KG
- 98.0%
- Shaanxi Dideu New Materials Co. Ltd
-

- Atogepant
1374248-81-3
- US $158.00-347.00 / mg
- 99.66%
- TargetMol Chemicals Inc.
-

- Atogepant
1374248-81-3
- US $0.00 / g
- 98% HPLC
- shandong perfect biotechnology co.ltd