Bis(1,5-cyclooctadien)nickel Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R11:Leichtentzündlich.
R40:Verdacht auf krebserzeugende Wirkung.
R45:Kann Krebs erzeugen.
S-S?tze Betriebsanweisung:
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S16:Von Zündquellen fernhalten - Nicht rauchen.
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.
Chemische Eigenschaften
Light yellow to Brown powder to crystal.
Verwenden
Bis(1,5-cyclooctadiene)nickel(0) is used as a catalyst for the cycloaddition of 1,3-dienes and is used to catalyze the addition of allyl phenyl sulfide to alkynes leading to 1,4-dienes. Also, it is involved as a catalyst for asymmetric alpha-arylation and heteroarylation of ketones with chloroarenes, stereoselective borylative ketone-diene coupling, cycloaddition of benzamides with internal alkynes and methyl carboxylation of homopropargylic alcohols.
synthetische
Bis(1,5-cyclooctadiene)nickel(0) is prepared by reduction of anhydrous nickel(II) acetylacetonate in the presence of the diolefin:
Ni(acac)2 + 2 cod + 2 AlEt3 → Ni(cod)2 + 2 acacAlEt2 + C2H6 + C2H4
Ni(cod)2 is moderately soluble in several organic solvents. One or both 1,5-cyclooctadiene ligands are readily displaced by phosphines, phosphites, bipyridine, and isocyanides. If exposed to air, the solid oxidizes to nickel(II) oxide. As a result, this compound is generally handled in a glovebox.
Reaktionen
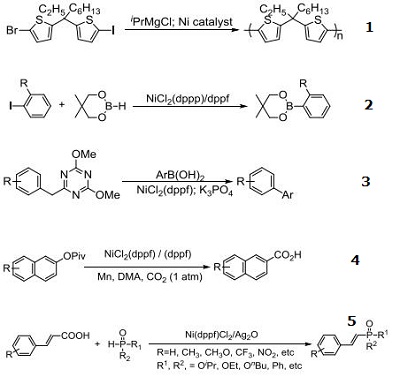
Catalyst for Grignard metathesis chain-growth polymerization of Poly(bithienylmethylene)s
Catalyst for neopentylglycolborylation of ortho-substituted aryl halides
Catalyst for Suzuki-Miyaura coupling reactions of heteroaryl ethers with arylboronic acids
Catalyst for carboxylation of naphthyl pivalates with CO2
Catalyst decarboxylative C?P cross-coupling of alkenyl acids with P(O)H compounds
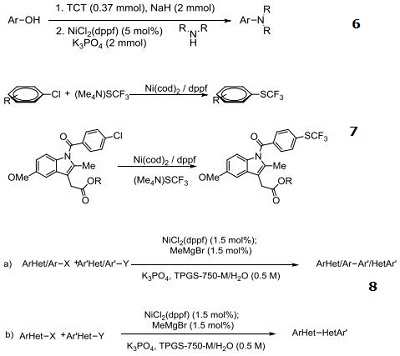
Catalyst for direct amination of phenols via C–O Bond Activation using 2,4,6-Trichloro-1,3,5-triazine as reagent
Catalyst for conversion of aryl, heteroaryl and pharmaceutically relevant chlorides to the corresponding trifluoromethyl sulphides
Catalytic precursor for Suzuki–Miyaura cross-coupling reactions in water under very mild reaction conditions: (a) aryl–heteroaryl cross-couplings; (b) Hetero–heteroaryl cross-couplings
Allgemeine Beschreibung
Yellow crystals or yellowish green solid.
Reaktivit?t anzeigen
BIS(1,5-CYCLOOCTADIENE)NICKEL(0) is extremely air and moisture sensitive. BIS(1,5-CYCLOOCTADIENE)NICKEL(0) is sensitive to exposure to light. BIS(1,5-CYCLOOCTADIENE)NICKEL(0) is incompatible with oxidizing agents, acids and acid fumes.
Brandgefahr
Flash point data for BIS(1,5-CYCLOOCTADIENE)NICKEL(0) are not available. BIS(1,5-CYCLOOCTADIENE)NICKEL(0) is probably combustible.
l?uterung methode
It is available in sealed ampoules under N2. All procedures should be carried out in a dry box and in an atmosphere of N2 or Argon in subdued light because the complex is light and oxygen sensitive, and flammable. The solid is washed with dry Et2O (under Ar) and separates from toluene as yellow crystals. Filter this under Ar gas pressure, place the crystals in a container and dry under a vacuum of 0.01mm to remove adhered toluene, flush with Ar and seal them under Ar or N2 in glass ampoules. [Semmelhack Org Reactions 19 115 and 178 1972, Wilke et al. Justus Liebigs Ann Chem 699 1 1966, Wender & Jenkins J Am Chem Soc 111 6432 1989, Fieser & Fieser Reagents for Org Synth 4 33, 16 29, 17 32.] SUSPECTED CARCINOGEN.
Bis(1,5-cyclooctadien)nickel Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

