Dimethylphosphonat Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE FLüSSIGKEIT.
PHYSIKALISCHE GEFAHREN
Die D?mpfe sind schwerer als Luft.
CHEMISCHE GEFAHREN
Schnelle Zersetzung beim Erhitzen unter Bildung giftiger Rauche mit Phosphoroxiden und Phosphin; bei Kontakt mit feuchter Luft über 220°C unter Bildung von Phosphors?ure und Methanol. Starke S?ure in w?ssriger L?sung. Reagiert sehr heftig mit Basen. ?tzend. Reagiert sehr heftig mit S?uren und Oxidationsmitteln.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).
MAK: Krebserzeugend Kategorie 3B; (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation, über die Haut und durch Verschlucken.
INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C tritt langsam eine gesundheitssch?dliche Kontamination der Luft ein.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Die Substanz reizt die Augen und die Haut.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
M?glich sind Auswirkungen auf die Augen mit nachfolgender Linsentrübung.
LECKAGE
Pers?nliche Schutzausrüstung: Atemschutzfilter für saure Gase. Chemikalienschutzanzug. NICHT in die Umwelt gelangen lassen. Verschüttetes Material mit Absorptionsmittel abdecken. Ausgelaufene Flüssigkeit in abdichtbaren Beh?ltern sammeln.
R-S?tze Betriebsanweisung:
R21:Gesundheitssch?dlich bei Berührung mit der Haut.
R36:Reizt die Augen.
R10:Entzündlich.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
Chemische Eigenschaften
colourless liquid
Verwenden
- Dimethyl Phosphite(DMP)is used as a reagent in the synthesis of 4-(thiophen-2-ylmethyl)-2H-phthalazin-1-ones as potent PARP-1 inhibitors.
- It is also used as a reagent in the synthesis of estafiatin phosphonate derivatives which exhibit antibacterial and antifungal activity.
- Dimethyl Phosphite is a degradation product of the pesticides trichlorphon and malathion and may be released into the envionment following their application. It is a contaminant (approxiately 2%) in the chemical intermediate triethyl phosphite, which hydrolyses readily to dimethyl hydrogen phosphite in the presence of moist air or water.
- Dimethyl Phosphite is used as a flame retardant on Nylon 6 fibres and, in combination with guanidine and formaldehyde, to impart flame and crease resistance to cotton textiles. The compound is also used to increase fire resistance to cellulosic textiles, acrolein-grafted polyamide fibres and y-irdiated polyethylene.
- lt is used as a lubricant additive, as a chemical intermediate in the production of organophosphorous pesticides and as an adhesive.
- Dimethyl Phosphite has also been used as a stabilizer in oil and plaster and, in combination with pyroctechol, as a corrosion inhibitor on steel.
Application
DMP is a basic chemical which is used industrially as an intermediate. Because of its reactivity
DMP participates in a large number of chemical reactions:
- Addition to oxo compounds
- Addition to oxo compounds with subsequent condensation e.g. with amines
- Oxidation with oxygen or chlorine
- Addition to alkenes
Due to these properties DMP is used as an intermediate for the manufacturing of
- water treatment chemicals e.g. corrosion inhibitors for cooling-water circuits (about 50 %)
- pesticides and pharmaceuticals (about 20 %)
- flame retardants and other specialities (about 15 %)
- textile finishing products (about 15 %)
Health Hazard
Dimethyl Phosphite (DMP) is rapidly absorbed via the oral and dermal routes. The main metabolic pathway in rodents is demethylation to monomethyl hydrogen phosphite (MMP) and further oxidation to CO2. DMP was mainly eliminated via urine and expired air. Over the studied dose range between 10 and 200 mg/kg bw and 5 x 200 mg/kg bw, respectively, only little evidence of bioaccumulation or saturation of absorption and elimination was observed. The only difference in studied toxicokinetics between rats and mice was the more rapid metabolism and elimination in mice.
An inhalation LC50 value is not available, but an exposure of 7100 mg/m³ (concentration estimated based on air flow and net loss of material) over 6 hours was not lethal for rats, mice and guinea pigs. Clinical signs were observed in mice only, and included occasionally laboured respiration after approximately 2 hours of exposure and ptosis after 5 hours. The acute dermal LD50 was 681 mg/kg bw (rabbits). Signs of intoxication were depression, ptosis, labored respiration, ataxia and placidity. The acute oral LD50 values were: 3283 mg/kg bw for male rats, 3040 mg/kg bw for female rats, 2815 mg/kg bw for male mice, and between 2150 and 3160 mg/kg bw for female mice. Clinical signs were inactivity, weakness, prostration and shallow breathing at doses near to or exceeding the LD50 values. White opaque eyes were seen in male mice.
Dimethyl Phosphite is irritating to the skin and eyes of rabbits. After prolonged or repeated exposures moderate to severe irritation of skin and mucosa was observed in rats. No sensitisation studies are available.
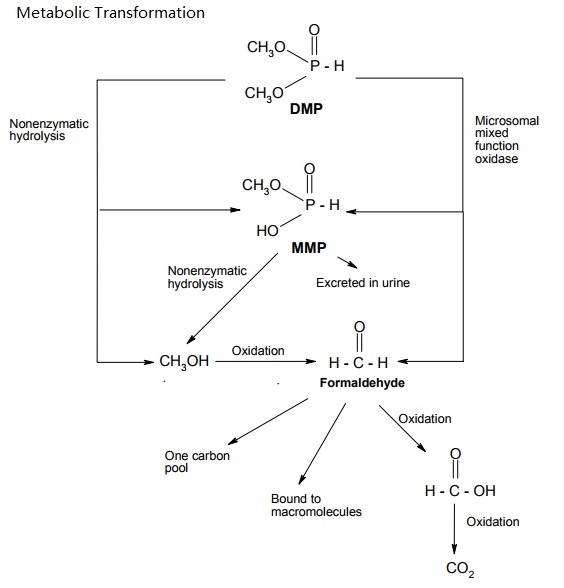
Proposed metabolic pathways of DMP in rats and mice (Nomeir and Matthews, 1997).
Sicherheitsprofil
Suspected carcinogen
with experimental carcinogenic data.
Moderately toxic by ingestion and skin
contact. A skin and eye irritant. Mutation
data reported. When heated to
decomposition it emits toxic fumes of POx
Carcinogenicity
Dimethyl hydrogen phosphite was not
mutagenic to several strains of Salmonella
typhimurium, but it did cause sister chromatid
exchanges and chromosomal aberrations in the
Chinese hamster CHO line.
An ACGIH threshold limit value (TLV)
has not been established for dimethyl hydrogen
phosphite.
Dimethylphosphonat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

