Isopren Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
LEICHT FLüCHTIGE FARBLOSE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH.
PHYSIKALISCHE GEFAHREN
Die D?mpfe sind schwerer als Luft und k?nnen sich am Boden ausbreiten. Fernzündung m?glich. Flie?en, Schütten o.?. kann zu elektrostatischer Aufladung führen.
CHEMISCHE GEFAHREN
Leichte Bildung explosionsf?higer Peroxide. Polymerisiert beim Erhitzen und unter Einfluss zahlreicher Materialien. Feuer- und Explosionsgefahr. Reagiert mit starken Oxidationsmitteln, starken Reduktionsmitteln, starken S?uren, starken Basen, S?urechloridenund Alkoholenunter Feuer- und Explosionsgefahr.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).
MAK nicht festgelegt (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation und durch Verschlucken.
INHALATIONSGEFAHREN
Nur ungenügende Angaben vorhanden über die Geschwindigkeit, mit der eine gesundheitssch?dliche Konzentration in der Luft beim Verdampfen bei 20°C erreicht wird.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Die Substanz reizt die Augen, die Haut und die Atemwege. M?glich sind Auswirkungen auf das Zentralnervensystem mit nachfolgender Atemdepression. Exposition kann Bewusstseinstrübung verursachen.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Risiko der Lungensch?digung bei wiederholter oder l?ngerer Exposition. M?glicherweise krebserzeugend für den Menschen.
LECKAGE
Gefahrenbereich verlassen! Fachmann zu Rate ziehen! Ausgelaufene Flüssigkeit m?glichst in abdichtbaren Beh?ltern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen. NICHT in die Kanalisation spülen. NICHT in die Umwelt gelangen lassen. Pers?nliche Schutzausrüstung: Vollschutzanzug mit umgebungsluftunabh?ngigem Atemschutzger?t.
R-S?tze Betriebsanweisung:
R45:Kann Krebs erzeugen.
R12:Hochentzündlich.
R52/53:Sch?dlich für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
R68:Irreversibler Schaden m?glich.
S-S?tze Betriebsanweisung:
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
Chemische Eigenschaften
Isoprene (2-methyl-l,3-butadiene) is a colorless, volatile, flammable liquid with specific gravity 0.6758. It is highly reactive, usually occurs as its dimer, and unless inhibited undergoes explosive polymerization. Isoprene naturally occurs in the environment as emissions from vegetation. It may be released to the environment as emissions during wood pulping, biomass combustion, and rubber abrasion; through tobacco smoke, gasoline, turbine, and automobile exhaust. In tobacco smoke, isoprene has been determined to be the precursor of a number of polycyclic aromatics, as demonstrated by thermal condensations in the range of 450–700℃.
Physikalische Eigenschaften
Colorless, volatile, extremely flammable liquid with an petroleum-like odor. An odor threshold concentration of 48 ppbV was reported by Nagata and Takeuchi (1990).
Verwenden
Isoprene occurs in nature and it is produced by many plants. Its polymers are the main component of natural rubber. The most important application of isoprene is to manufacture polymers and copolymers. Polyisoprene, a synthetic rubber made from isoprene, is used in a wide variety of rubber applications including medical equipment, baby bottle teats/nipples, toys, shoe soles, tires, elastic films, threads for golf balls or textiles, adhesives, paints, and coatings. Copolymer butyl rubber, made from isobutene with a small amount of isoprene, has excellent impermeability to gases and is used in inner tubes. Another copolymer styrene-isoprene rubber is used in pressure sensitive adhesives. Isoprene is also used as a chemical intermediate.
synthetische
Isoprene is obtained from propylene by the followin,g route:
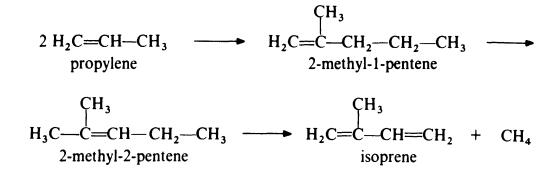
In the first step, propylene is dimerized to 2-methyl-l-pentene by passage
over a catalyst of tri-n-propylaluminium at about 200??C and 20 MPa (200
atmospheres). This product is then isomerized to 2-methyl-2-pentene by
heating at 150-300??C in the presence of a silica-alumina catalyst. The final
step in the process is the pyrolysis of the olefin to isoprene at 650-800??C in
the presence of a free radical initiator such as hydrogen bromide. The
isomerization step is necessary because pyrolysis of 2-methyl-l-pentene gives
much poorer yields of isoprene than pyrolysis of 2-methyl-2-pentene.
Vorbereitung Methode
Rubber results from the polymerization of isoprene to form polyisoprene. The resultingstructure dictates the properties of the rubber. Natural rubber has a cis 1,4 structure.This means that the carbon atoms that form the chainattach to the same side ofthe chain at the 1 and 4 positions. The cisstructure gives rubber its elasticity. Polyisoprene alsoexists in a trans 1,3 configuration. In the trans configuration, the addition takes place onopposite sides of the carbon chain.
Natural rubber occurs in a colloidal milky suspension called latex, which is obtained fromnumerous plants. The most important of these is the para rubber tree, Hevea brasiliensis. Naturalrubber is harvested by cutting a v-shape incision into a plant and allowing latex to drain intoa container containing a preservative. About 50mL of latex is obtained on a daily basis. Latexis transported to collection stations where it is processed for shipment. Processing can includepreservation, coagulation, and concentrating before being sent to rubber factories.
Definition
ChEBI: A hemiterpene with the formula CH22C(CH3)CH2CH2; the monomer of natural rubber and a common structure motif to the isoprenoids, a large class of other naturally occurr
ng compounds.
Allgemeine Beschreibung
A clear colorless liquid with a petroleum-like odor. Density 5.7 lb / gal. Flash point -65°F. Boiling point 93°F. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a closed container, the container may rupture violently. Less dense than water and insoluble in water. Vapors heavier than air.
Air & Water Reaktionen
Highly flammable. Insoluble in water.
Reaktivit?t anzeigen
ISOPRENE may react vigorously with strong oxidizing agents. May react exothemically with reducing agents to release hydrogen gas. May undergo exothermic addition polymerization in the presence of various catalysts (such as acids) or initiators. Undergoes autoxidation upon exposure to the air to form explosive peroxides. Mixing isoprene in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid (70%), oleum, sulfuric acid (90%) [NFPA 1991].
Hazard
Highly flammable, dangerous fire and
explosion risk. Irritant. Possible carcinogen.
Health Hazard
Vapor produces no effects other than slight irritation of the eyes and upper respiratory tract. Liquid may irritate eyes; like gasoline.
Carcinogenicity
Isoprene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
At 25 ℃, isoprene has a high vapor pressure of 733 hPa, a low
water solubility of 642 mg l
-1, and a Henry’s law constant of
7781 Pam
3 mol
-1. Isoprene’s log Kow is 2.42 while its log Koc
is 1.83. Isoprene’s vapor density relative to air is 2.4. Because of
its high vapor pressure at ambient temperature, isoprene will
partition largely into the atmosphere, with negligible amounts
partitioning to soil and water. Due to a short half-life in air
(0.5 h by reaction with nitric oxide, 1.2–4 h by reaction with
hydroxyl radicals, and 19 h by reaction with ozone), wet
deposition of isoprene from air is not expected to play
a significant role in its atmospheric fate. Although laboratory
testing demonstrates that isoprene has the potential to biodegrade,
microbial metabolism is unlikely to contribute significantly
to the removal of isoprene from the environment due to
rapid volatilization from terrestrial and aquatic media.
Isoprene has a low bioaccumulation potential and is not
expected to bioaccumulate.
l?uterung methode
Reflux it with sodium then distil it from sodium or NaBH4 under nitrogen, and pass it through a column containing KOH, CaSO4 and silica gel. tert-Butylcatechol (0.02% w/w) is added, and the isoprene is stored in this way until redistilled before use. The inhibitor (tert-butylcatechol) in isoprene can be removed by several washings with dilute NaOH and water. The isoprene is then dried over CaH2, distilled under nitrogen at atmospheric pressure, and the fraction distilling at 32o is collected. Store it under nitrogen at -15o. [Beilstein 1 H 252, 1 IV 1001.]
Isopren Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
2,2-DIMETHYLCYCLOPENTANONE
3-Methyl-2,5-dihydrothiophen-1,1-dioxid
6-Methylhept-5-en-2-on
3-Methyl-2-buten-1-ol
butyl rubber
2-Methylbut-2-en
Ionon, Methyl-
Cyclopenten
TERPINEOL
1-Chlor-3-methylbut-2-en
Methyl tetrahydrophthalic anhydride
3-Methylbutanon
3,7-Dimethyl-1,7-octadien-3-ol
2,6-Dimethyl-1,7-octadien-3-ol
3,6-Dihydro-4-methyl-2-(2-methylpropyl)-2H-pyran
DECANE,3,8-DIMETHYL-

