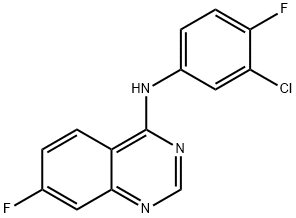
Afatinib Impurity
|
|
Afatinib Impurity Eigenschaften
- Siedepunkt:
- 403.9±45.0 °C(Predicted)
- Dichte
- 1.485±0.06 g/cm3(Predicted)
- pka
- 5.07±0.30(Predicted)
Sicherheit
Afatinib Impurity Chemische Eigenschaften,Einsatz,Produktion Methoden
Afatinib Impurity Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Afatinib Impurity Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 6)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 |
overseasales@yongstandards.com | China | 11922 | 58 |
| ShenZhen H&D Pharmaceutical Technology Co., LTD | +8618627948422 |
sale@hdimpurity.com | China | 3389 | 58 |
| QUALITY CONTROL SOLUTIONS LTD. | 0755-66853366; 13670046396 |
ORDERS@QCSRM.COM | China | 24342 | 58 |
| Wuhan Yanzhe Technology Co., Ltd | 15527250409 |
wenhaotian12@163.com | China | 4148 | 58 |
| HX Pharmaceutical technology co., LTD | 15671363360 |
494267389@qq.com | China | 3523 | 58 |
| ShenZhen H&D Pharmaceutical Technology Co., LTD | 13217415044 13217415044 |
2851922763@qq.com | China | 7933 | 58 |
- 4-Quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-fluoro-
- Afatinib Impurity 87
- 1401162-04-6