Lithiumchromat Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R8:Feuergefahr bei Berührung mit brennbaren Stoffen.
R43:Sensibilisierung durch Hautkontakt m?glich.
R49:Kann Krebs erzeugen beim Einatmen.
R50/53:Sehr giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
S-S?tze Betriebsanweisung:
S17:Von brennbaren Stoffen fernhalten.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.
S60:Dieses Produkt und sein Beh?lter sind als gef?hrlicher Abfall zu entsorgen.
Chemische Eigenschaften
Lithium chromate is a yellow or orange-yellow crystalline powder. It dissolves in strong acids and alkalis but is insoluble in water and oil. It is made from lithium hydroxide and chromic acid.

Verwenden
Lithium Chromate is used in preparation method method of permeable crystalline cement-based waterproof coating by using maifanstone composite high water-absorbing resin and its application in building.
synthetische
According to the following equations, Li2CrO4 is formed on boiling a solution of (NH4)2Cr2O7 with LiOH.
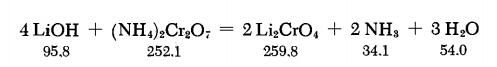
Allgemeine Beschreibung
Lithium chromate appears as a yellow crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It is used as a corrosion inhibitor and in the manufacture of other chemicals.
Air & Water Reaktionen
Deliquescent. Water soluble
Reaktivit?t anzeigen
Lithium chromate is an oxidizing agent. Can oxidize combustibles [USCG, 1999]. Combining the chromate with zirconium can be explosive given the right proportions of reactants, [Z. Anorg., 1930, 191, 113].
Health Hazard
INHALATION: Corrosive to skin and mucous membranes causing dermatitis and slow healing ulcers. EYES: Conjunctivitis and lacrimation. INGESTION: Violent gastroenteritis, peripheral vascular collapse, vertigo, muscle cramps, coma, hemorrhagic diathesis, fever, liver damage and renal failure.
m?gliche Exposition
Used as a corrosion inhibitor, heat
transfer agent; and oxidizing agent in leather and metal finishing. Also used in photography, wood preservatives; batteries, safety matches, and cement
Versand/Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
Inkompatibilit?ten
Aqueous solution is caustic. A strong
oxidizer; strong reaction with reducing agents, combustibles, organic material, hydrazine, chromic acid; sulfur,
acids. Attacks plastics and aluminum
Lithiumchromat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

