Melamin Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE BIS WEISSE KRISTALLE
PHYSIKALISCHE GEFAHREN
Staubexplosion der pulverisierten oder granulierten Substanz in Gemischen mit Luft m?glich.
CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen oder beim Verbrennen unter Bildung von giftigen und reizenden Stoffen einschlie?lich Cyanwasserstoff, Stickoxide und Ammoniak.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt.
MAK nicht festgelegt.
INHALATIONSGEFAHREN
Eine bel?stigende Partikelkonzentration in der Luft kann schnell erreicht werden beim Dispergieren, vor allem als Pulver.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Nach Verschlucken gr??erer Mengen: M?glich sind Auswirkungen auf die Nieren und die Blase mit nachfolgender Bildung von Steinen.
LECKAGE
Pers?nliche Schutzausrüstung: Atemschutzger?t, P2-Filter für sch?dliche Partikel. Verschüttetes Material in verschlie?baren Beh?ltern sammeln; falls erforderlich durch Anfeuchten Staubentwicklung verhindern. Reste sorgf?ltig sammeln. An sicheren Ort bringen.
R-S?tze Betriebsanweisung:
R43:Sensibilisierung durch Hautkontakt m?glich.
R44:Explosionsgefahr bei Erhitzen unter Einschluss.
R20/21:Gesundheitssch?dlich beim Einatmen und bei Berührung mit der Haut.
S-S?tze Betriebsanweisung:
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
Beschreibung
Melamine-formaldehyde resin (MFR) is an active
ingredient of strong (reinforced) plasters. Sensitization
was reported in a plaster-room technician, who applied
resin-reinforced pIaster casts, and in dental technicians.
MFR was contained in a strong dental pIaster
used for mouldings. Used as a textile finish res in, it was
also found to be an allergen in a women who replaced
clothes in a store. MFR also releases formaldehyde,
which may be the sensitizer.
Chemische Eigenschaften
Melamine is a white solid organic compound whose molecules consist of a sixmembered heterocyclic ring of alternate carbon and nitrogen atoms with three amino groups attached to the carbons. Condensation polymerization with methanal or other aldehydes produces melamine resins, which are important thermosetting plastics.
Verwenden
It is used to make high-pressure laminating resins
(e.g., decorative countertops), molded compounds (e.g.,
dinnerware), and surface coating resins (e.g., appliance
finishes and automotive topcoats). Additional major products
are textile and paper treatment resins. Miscellaneous uses
include adhesive resins for gluing lumber, plywood, and
flooring, and resins for leather tanning agents. Melamine,
melamine cyanurate, other melamine salts, and guanidine
compounds are currently the most used group of nitrogencontaining
flame retardants. Melamine is used as a flame
retardant additive for polypropylene and polyethylene.
Melamine cyanurate is employed commercially as a flame
retardant for polyamides and terephthalates.
Vorbereitung Methode
Melamine is prepared almost exclusively by the urea
process—the action of ammonia on urea. It is produced
worldwide.
synthetische
The standard route to melamine is from urea. Urea is
heated in the presence of ammonia at 250-350??C and 4--20 MPa. The
reaction probably involves the simultaneous dehydration and hydration of
urea to form cyanamide and ammonium carbamate; trimerization of the
cyanamide then leads to melamine:
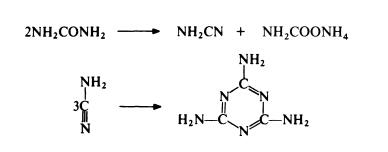
Thus only 50% of the urea used gives melamine in one step and ammonium
carbamate has to be separated and converted to urea for recycling. Despite
this limitation, the urea route is the most economical of currently available
routes.
Allgemeine Beschreibung
Colorless to white monoclinic crystals or prisms or white powder. Sublimes when gently heated.
Air & Water Reaktionen
Insoluble in water.
Reaktivit?t anzeigen
Melamine is incompatible with strong oxidizing agents and strong acids . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Hazard
Toxic by ingestion, skin, and eye irritant.
Questionable carcinogen.
Brandgefahr
Literature sources indicate that Melamine is nonflammable.
Kontakt-Allergie
Melamine-formaldehyde resin (MFR) results from condensation of melamine and formaldehyde. It is anactive ingredient of strong (reinforced) plasters, such as industrial or some dental plasters used for molding.It is also used as a textile finish resin. MFR acts as an allergen generally because of formaldehyde releasing (see Chap. 40)
Sicherheitsprofil
Moderately toxic by
ingestion and intraperitoneal routes. An eye,
skin, and mucous membrane irritant. Causes
dermatitis in humans. Questionable
carcinogen with experimental carcinogenic
and tumorigenic data. Experimental
reproductive effects. Mutation data
reported. When heated to decomposition it
emits toxic fumes of NOx and CN-.
m?gliche Exposition
Manufactured from urea, melamine
is used in the manufacture of plastics, melamineformaldehyde resins; rubber, synthetic textiles; laminates,
adhesives, and molding compound
Carcinogenicity
A bioassay of melamine was
conducted in rats and mice by NTP. Male F344 rats and
B6C3F1 mice were administered melamine in their diets at
concentrations of 2250 or 4500 ppm daily for 103 weeks.Female rats were fed 4500 or 9000 ppm melamine. At the end
of 111 weeks, surviving animals were killed and examined.
l?uterung methode
Crystallise Melamine from water or dilute aqueous NaOH. It sublimes at ~240o on prolonged heating. [Beilstein 26 I 74, 26 II 132, 26 III/IV 1253.]
Inkompatibilit?ten
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Melamine neutralizes
acids in exothermic reactions to form salts plus water. May
be incompatible with isocyanates, halogenated organics,
peroxides, phenols (acidic), epoxides, anhydrides, and acid
halides. Flammable gaseous hydrogen may be generated in
combination with strong reducing agents such as hydrides,
nitrides, alkali metals, and sulfides.
Melamin Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Early-strength admixture
water proofing agent 703
softener PEG
Fluorescent Brightener BC
REACTIVE GREEN 19
1-Amino-4-(2'-(4',6'-dichlor-s-triazin-2-yl)amino)phenylamino)9,10-dihydro-9,10-dioxoanthracen-2,4'-disulfonsaeure
Various color amino baking enamel A04-9
demulsifier KN-1
modified phenol-formaldehyde resin
Pumping agent
Amino resin varnish
finishing agent KB for polyester viscose blend
anchorage used for glassine paper
PAINT
MELAMINE RESIN
Amino resin paint
conductive coating-composite system of acrylic copolymer and cuprous iodide
Slushing agent,high efficiency
Amino moulding plastic
3,4,5,6-Tetrachlor-2-(1,4,5,8-tetrabrom-6-hydroxy-3-oxoxanthen-9-yl)benzoesure
Trimethylolmelamine Resin
Bornitrid

