Penicillamin Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R40:Verdacht auf krebserzeugende Wirkung.
R20/21/22:Gesundheitssch?dlich beim Einatmen,Verschlucken und Berührung mit der Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S24/25:Berührung mit den Augen und der Haut vermeiden.
S22:Staub nicht einatmen.
Beschreibung
Penicillamine is an orally bioavailable copper chelator and penicillin degradation product. It increases urinary and fecal copper excretion and decreases liver copper concentration in a rat model of copper overload when administered at 0.67 mmol/kg per day, but does not affect kidney, spleen, or brain copper levels. Penicillamine (100 mg/kg per day) dissolves copper-rich granules in hepatic lysosomes of Long-Evans cinnamon (LEC) rats, which spontaneously develop hepatic injury and acute hepatitis and have a mutation homologous to that of the human Wilson disease gene. Penicillamine has anticonvulsant and proconvulsant effects in mice when administered at 0.5 and 250 mg/kg, respectively, which are blocked by the nitric oxide synthase (NOS) inhibitors L-NAME and 7-nitroindazole . Formulations containing penicillamine have been used to treat Wilson disease, cystinuria, and active rheumatoid arthritis.
Chemische Eigenschaften
White to off-white crystalline powder
Verwenden
As a Penicillin metabolite, D-(-)-Penicillamin can be used in the treatment of Wilson’s disease, Cystinuria, Scleroderma and arsenic poisoning.
Definition
ChEBI: An optically active form of penicillamine having D-configuration. Pharmaceutical form (L-form is toxic) of chelating agent used to treat heavy metal poisoning.
Allgemeine Beschreibung
D-Penicillamine contains a β-lactam chemical structure.
Sicherheitsprofil
Poison by
intraperitoneal route. Moderately toxic by
subcutaneous and intravenous routes. Mildly
toxic by ingestion. An experimental
teratogen. Human systemic effects by
ingestion: agranulocytosis, dermatitis, fever,
hemorrhage, increased body temperature,
dermatitis, leukopenia, proteinuria,
thrombocytopenia. Human teratogenic
effects by an unspecified route:
developmental abnormalities of the
craniofacial areas, skin, and skin appendages,
and body wall. Experimental reproductive
effects. Questionable human carcinogen
producing leukemia. Mutation data reported.
Used in the treatment of rheumatoid
arthritis, metal poisonings, and cystinuria.
When heated to decomposition it emits very
toxic fumes of NOx and SOx. See also
MERCAPTANS.
Synthese
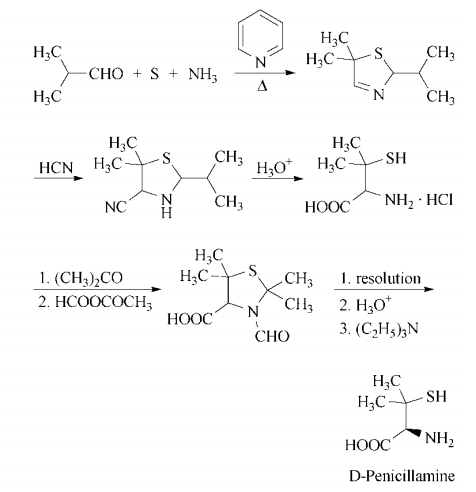
d-penicillamine can be synthesized in a multistep process that begins with
heating isobutyraldehyde, pyridine, sulfur, and
ammonia in benzene to form 5,5-dimethyl-2-
isopropyl-?3-thiazoline. Treatment with hydrogen cyanide gives 4-cyano-5,5-dimethyl-
2-isopropylthiazolidine, which on acid hydrolysis gives d,l-penicillamine hydrochloride. Resolution is accomplished by
conversion of the racemate to d,l-3-formyl-
2,2,5,5-tetramethylthiazolidine-4-carboxylic
acid by treatment first with acetone, then
with acetic formic anhydride. The enantiomers are separated in the usual manner,
using, for example, l-lysine or d-(?)-
threo-1-(4-nitrophenyl)-2-aminopropane-1,3-
diol. Acidification liberates d-3-formyl-
2,2,5,5-tetramethylthiazolidine-4-carboxylic
acid, which is hydrolyzed with hydrochloric
acid to yield d-penicillamine hydrochloride.
Neutralization with ethanolic triethylamine affords d-penicillamine.
l?uterung methode
The melting point of D-(-)-penicillamine depends on the rate of heating (m 202-206o is obtained by starting at 195o and heating at 2o/minute). It is soluble in H2O and alcohols but insoluble in Et2O, CHCl3, CCl4 and hydrocarbon solvents. Purify it by dissolving it in MeOH and adding Et2O slowly. Dry it in vacuo and store it under N2. [Weight et al. Angew Chem, Int Ed (English) 14 330 1975, Cornforth in The Chemistry of Penicillin (Clarke, Johnson and Robinson eds) Princeton Univ Press, 455 1949, Review: Chain et al. Antibiotics (Oxford University Press) 2 1949, Polymorphism: Vidler J Pharm Pharmacol 28 663 1976]. The D-S-benzyl derivative has m 197-198o (from H2O), [] D 17 -20o (c 1, N NaOH), -70o (N HCl). [Beilstein 4 IV 3228.]
Penicillamin Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

