Caesiumbromid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Beschreibung
Cesium bromide is a colorless crystal with the formula CsBr. It is a moderately water soluble crystalline Cesium source that decomposes to Cesium oxide on heating. Most metal bromine compounds are water-soluble and are used in water treatment, chemical analysis, and ultra-high purity for certain crystal growth applications.
Chemische Eigenschaften
Cesium bromide is a colourless crystals. It has been used in protein crystallization for anamolous scattering.

Verwenden
Cesium bromide (CsBr) crystals are used in scintillation counters to detect radiation. The
compound is also used to coat the inside of fluorescent screens.
synthetische
Caesium bromide can be prepared via following reactions:
Neutralization:
CsOH (aq) + HBr (aq) → CsBr (aq) + H2O (l)
Cs2(CO3) (aq) + 2 HBr (aq) → 2 CsBr (aq) + H2O (l) + CO2 (g)
Direct synthesis:
2 Cs (s) + Br2 (g) → 2 CsBr (s)
The direct synthesis is a vigorous reaction of caesium with other halogens. Due to its high cost, it is not used for preparation.
Sicherheitsprofil
Moderately toxic by
intraperitoneal route. See also CESIUM and
BROMIDES. When heated to
decomposition it emits toxic fumes of Br-.
l?uterung methode
It is very soluble in H2O, soluble in EtOH but insoluble in Me2CO. Dissolve it in the minimum volume of H2O, filter and precipitate it by adding Me2CO. Filter off the solid and dry it at 100o. Also recrystallise it from water (0.8mL/g) by partial evaporation in a desiccator.
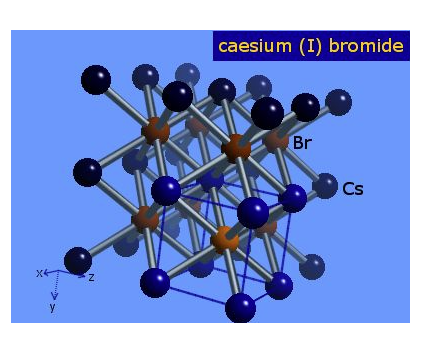
Structure and conformation
The space lattice of CsBr(Cesium bromide) belongs to the cubic system, and its cesium chloride structure has a lattice constant of a=0.429 nm.
Caesiumbromid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

