Buttersureanhydrid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R14:Reagiert heftig mit Wasser.
R34:Verursacht Ver?tzungen.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
Beschreibung
Butyric anhydride, or Butanoic anhydride, is a chemical compound with the formula (CH
3CH
2CH
2CO)
2O.It is a colorless liquid that smells strongly of butyric acid, formed by its reaction with the moisture in the air.
Chemische Eigenschaften
clear liquid
Verwenden
Butyric anhydride is used in the preparation of amidoamine dendron-based co-adsorbents, which finds application in dye- sensitized solar cells improvement. It is also used in the synthesis of butyrate ester, which is used as a perfume and flavor. Further, it acts as a fumigant to drive bees from their hives. In addition to this, it is used in food additives, textile auxiliaries, varnishes, perfumes, pharmaceuticals and disinfectants.
Application
Because of its odor, butyric anhydride has use as a fumigant to drive bees from their hives in products such as Bee - Go.
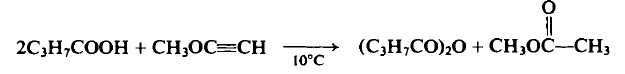
synthetische
To a cooled flask containing 88 gm (1.0 mole) of η-butyric acid at 10°C is added dropwise 28.0 gm (0.5 mole) of methoxyacetylene over a 1-hr period. After stirring the reaction mixture for 16 hr at 20°C the contents are distilled to afford the following three fractions: (1) b.p. 56°C, η& 1.3628, 23 gm (62%), methyl acetate; (2) b.p. 72-73°C (18 mm Hg), n% 1.3990, 10.0 gm (11%), butyric acid; (3) b.p. 91-92°C (18mmHg), 1.4118, 48.0 gm (61%), butyric anhydride.
Allgemeine Beschreibung
Water-white liquid with an odor of rancid butter. Flash point 190°F. Density 8.0 lb / gal. Corrosive to metals and tissue. Low toxicity.
Air & Water Reaktionen
Slowly reacts with water to form butyric acid.
Reaktivit?t anzeigen
Butyric anhydride reacts exothermically with water. The reaction is usually slow, but might become violent if local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Brandgefahr
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Sicherheitsprofil
Mildly toxic by
ingestion. A corrosive liquid. When heated
to decomposition it emits acrid smoke and
irritating vapors.
Sicherheit(Safety)
Butyric anhydride is a combustible, corrosive liquid. It is considered water sensitive.
l?uterung methode
Dry the anhydride by shaking it with P2O5, then distilling it. [Beilstein 2 IV 802.]
Buttersureanhydrid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

