Diallylphthalat Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE FLüSSIGKEIT
CHEMISCHE GEFAHREN
Der Stoff kann polymerisieren beim Erhitzen oder in Gegenwart eines Katalysators, wenn nicht inhibiert. Beim Verbrennen Bildung von giftigen Gasen. Reagiert mit starken Oxidationsmitteln, S?uren und Basen.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt.
MAK: IIb (nicht festgelegt, aber Informationen vorhanden) (DFG 2006).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation des Aerosols.
INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C tritt langsam eine gesundheitssch?dliche Kontamination der Luft ein, viel schneller jedoch beim Versprühen oder Dispergieren.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Verschlucken der Flüssigkeit kann zur Aufnahme in der Lunge führen; Gefahr der Aspirationspneumonie.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Wiederholter oder andauernder Kontakt kann zu Hautsensibilisierung führen.
LECKAGE
Belüftung. Ausgelaufene Flüssigkeit in abgedeckten Beh?ltern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen. NICHT in die Umwelt gelangen lassen.
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.
R50/53:Sehr giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
S-S?tze Betriebsanweisung:
S24/25:Berührung mit den Augen und der Haut vermeiden.
S60:Dieses Produkt und sein Beh?lter sind als gef?hrlicher Abfall zu entsorgen.
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
Chemische Eigenschaften
clear colourless to light yellow liquid
Verwenden
Diallyl Phthalate is used as a reagent in ring-closing ruthenium based reactions.
synthetische
Diallyl phthalate (DAP) is prepared by reaction of phthalic anhydride and allyl alcohol:
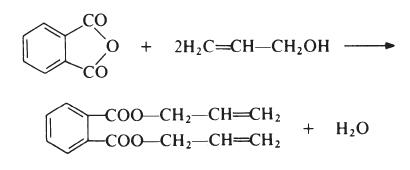
Application
Diallyl phthalate is an important monomer for the production of thermosetting molding compounds, which must have good dimensional stability and electrical properties, and be resistant to heat and solvents. Diallyl phthalate can be polymerized or copolymerized. This usually is done by dissolving the diallyl phthalate monomer in 2- propanol, adding 50% hydrogen peroxide at about 105 ℃, and precipitating the prepolymer from the cooled, viscous solution with excess 2- propanol. Copolymers containing diallyl phthalate are suitable for specialty coating and for embedding, especially in the production of electronic devices. For example, the moisture-sensitive epoxy compounds now used in light-emitting diode (LED) displays can be replaced by stable diallyl phthalate epoxy encapsulating resins. By adding inorganic materials to diallyl phthalate prepolymer compositions, reinforced thermosetting molding compounds can be obtained. Glass cloth or paper can be impregnated with a solution of prepolymer, monomer, and peroxide initiator. After removal of the solvent, the glass cloth or paper is cured to give the desired film-protected material, which is used for decoration, stain-resistant overlays for household articles, and furniture.
Allgemeine Beschreibung
Clear pale-yellow liquid. Odorless.
Air & Water Reaktionen
Incompatible with water and oxygen. Should be stored air tight, with inhibitor, to prevent polymerization reaction .
Reaktivit?t anzeigen
Diallyl phthalate can react with oxidizers. Diallyl phthalate can also react with acids and alkalis. Diallyl phthalate is incompatible with water and oxygen.
Brandgefahr
Diallyl phthalate is combustible.
Sicherheitsprofil
Suspected carcinogen
with experimental carcinogenic data.
Moderately toxic by ingestion, skin contact,
intraperitoneal, and subcutaneous routes. An
eye irritant. Mutation data reported.
Combustible when exposed to heat or
flame; can react with oxidzing materials. To
fight fire, use CO2 or dry chemical. When
heated to decomposition it emits acrid
smoke and irritating fumes. See also ALLYL
COMPOUNDS and ESTERS.
Carcinogenicity
In the 103-week study referred
to previously, a slight increase in MNCL was seen in
female rats treated with 50 or 100 mg/kg/day of DAP. MNCL
occurs in F344 control rats at a high incidence; however, the
incidence of 51% in female rats at the high dose level was
above historical control data for the laboratory (29%). No
significant increases in tumor incidences were seen in male
rats. Based on this study, DAP was considered to have
demonstrated equivocal evidence for carcinogenicity in
female F344 rats according to the NTP.
In male and female B6C3F1 mice receiving 300 mg/kg of
DAP by gavage for 103 weeks (5 days/week), the incidence
of forestomach papillomas was significantly greater than that
of controls. Because of the rarity of forestomach
papillomas in control B6C3F1 mice and the concomitant
observation of dose-related forestomach hyperplasia, the
development of these tumors was considered to be test
substance related. Compared to controls, a slight increase
in the incidence of lymphomas was observed in males
receiving 300 mg/kg/day of DAP. Because the increase
was not statistically significant compared to historical control
data, this effect was not considered to be test substance
related.
Diallylphthalat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

