| Identification | More | [Name]
Benzoyl peroxide | [CAS]
94-36-0 | [Synonyms]
BENZOPEROXIDE
BENZOYL BENZENECARBOPEROXOATE
BENZOYL DIOXIDE
BENZOYL PEROXIDE
Benzoyl superoxide
DIBENZOYL PEROXIDE
LUPEROX A70S
LUPEROX A75
LUPEROX A75FP
LUPEROX AFR40
LUPEROX ATC50
Perkadox L
VAROX ANS
Abcure S-40-25
Acetoxyl
AcetOxyl 2.5 and 5
Acne-Aid Cream
Acnegel
Akneroxid 5
Akneroxid L | [EINECS(EC#)]
202-327-6 | [Molecular Formula]
C14H10O4 | [MDL Number]
MFCD00003071 | [Molecular Weight]
242.23 | [MOL File]
94-36-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Benzoyl peroxide is an odorless, white or colorless
crystalline powder. | [Melting point ]
105 °C(lit.)
| [Boiling point ]
176°F | [density ]
1.16 g/mL at 25 °C(lit.)
| [vapor pressure ]
0.009Pa at 25℃ | [refractive index ]
1.5430 (estimate) | [Fp ]
>230 °F
| [storage temp. ]
2-8°C
| [solubility ]
0.35mg/l | [form ]
powder
| [color ]
White | [Odor]
odorless | [Stability:]
Strong oxidizer. Highly flammable. Do not grind or subject to shock or friction. Incompatible with reducing agents, acids, bases, alcohols, metals, organic materials. Contact with combustible material, heating or friction may cause fire or explosion. | [Water Solubility ]
Insoluble | [Merck ]
14,1116 | [BRN ]
984320 | [Exposure limits]
TLV-TWA 5 mg/m3; IDLH 7000 mg/m3. | [Contact allergens]
Benzoyl peroxide is an oxidizing agent widely
employed in acne topical therapy. It is also used as a
polymerization catalyst of dental or industrial plastics
and as a decolorizing agent of flours, oils, fats, and
waxes. Irritant or allergic dermatitis may affect workers
in the electronics and plastics (epoxy resins and
catalysts) industries, electricians, ceramic workers,
dentists and dental technicians, laboratory technicians,
bakers, and acne patients. As it was contained in candles,
it also induced contact dermatitis in a sacristan.
Patch tests may be irritant. | [InChIKey]
OMPJBNCRMGITSC-UHFFFAOYSA-N | [LogP]
3.2 at 20℃ | [Uses]
Benzoyl Peroxide is a colorless, crystalline solid with a faint odor of
benzaldehyde resulting from the interaction of benzoyl chloride
and a cooled sodium peroxide solution. it is insoluble in water. it is
used in specified cheeses at 0.0002% of milk level. it is used for the
bleaching of flour, slowly decomposing to exert its full bleaching
action, which results in whiter flour and bread. | [CAS DataBase Reference]
94-36-0(CAS DataBase Reference) | [IARC]
3 (Vol. 36, Sup 7, 71) 1999 | [NIST Chemistry Reference]
Benzoyl peroxide(94-36-0) | [EPA Substance Registry System]
94-36-0(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
white powder or crystals | [Uses]
Benzoyl Peroxide is a widely used organic compound of the peroxide family. Benzoyl Peroxide is often used in acne treatments , bleaching and polymerizing polyester and many other uses. | [Uses]
vitamin B complex | [Uses]
Source of free radicals for industrial processes. Oxidizing agent in bleaching oils, flour, etc.; catalyst in the plastics industry; initiator in polymerization. | [General Description]
White, odorless powder, moderately toxic. | [Reactivity Profile]
BENZOYL PEROXIDE is most dangerous when BENZOYL PEROXIDE, [> 77% BUT < 95% WITH WATER] contains less then 1% water. A moisture content of 3% allowed slow burning only, and at 5% ignition did not occur [McCloskey, C. M. et al., Chem. Abs., 1967, 66, 12613c]. Mixed with a large surplus of water (30% or more) BENZOYL PEROXIDE, [> 77% BUT < 95% WITH WATER] is relatively safe. In dry form BENZOYL PEROXIDE, [> 77% BUT < 95% WITH WATER] is a very dangerous material; BENZOYL PEROXIDE, [> 77% BUT < 95% WITH WATER] will explode spontaneously when heated above melting point (103° C). An explosion which occurred when a screw-capped bottle of the peroxide was opened was attributed to friction initiating a mixture of peroxide Ind organic dust in the cap threads [Lappin, G. R., Chem. Eng. News, 1948, 26, p.3518]. A violent explosion occurred during purification of the peroxide by Soxhlet extraction with hot chloroform [Anon., Sichere Chemiearb., 1976, 28, p. 49]. BENZOYL PEROXIDE, [> 77% BUT < 95% WITH WATER] is a powerful oxidizer, BENZOYL PEROXIDE, [> 77% BUT < 95% WITH WATER] ignites readily and burns rapidly. In contact with reducing agents BENZOYL PEROXIDE, [> 77% BUT < 95% WITH WATER] may ignite by spontaneous chemical reaction. BENZOYL PEROXIDE, [> 77% BUT < 95% WITH WATER] must be kept in a cool place in isolation, out of the sunlight or sources of heat, avoid shock or friction. BENZOYL PEROXIDE, [> 77% BUT < 95% WITH WATER] reacts violently with inorganic or organic acids, alcohols, amines, metallic naphthenates and polymerization accelerators (e.g., N,N-dimethylaniline). Explosive or violent reaction on contact with dimethyl sulfide, lithium aluminum hydride or aniline [Bretherick, 5th ed., 1995, p. 1140]. Mixture with carbon tetrachloride and ethylene explodes when exposed to heat [Bolt, R. O. et al., Chem. Eng. News, 1947, 25, p. 1866]. Ignition occurred on contact with methyl methacrylate [MCA Case History No. 996], polymerization of vinyl acetate in ethyl acetate accelerated out of control leading to ignition and explosion [Vervalin, 1973, p. 81]. At 50° C a mixture of dibenzoyl peroxide and charcoal reacts violently producing dense white smoke of benzoic acid, benzene, phenyls and carbon dioxide [Leleu, Cahiers, 1980, 99, p. 279]. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Highly toxic via inhalation. May explode
spontaneously when dry (<1% of water). Never mix
unless at least 33% water is present. Skin and upper
respiratory tract irritant. Questionable carcinogen.
| [Potential Exposure]
Used as polymerization initiator, curing
agent, and cross-linking agent. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Medical observation is recommended for 24 to 48 hours
after breathing overexposure, as pulmonary edema may be
delayed. As first aid for pulmonary edema, a doctor or
authorized paramedic may consider administering a drug or
other inhalation therapy. | [Shipping]
UN3104 : Organic peroxide type C, solid, Hazard
Class: 5.2; Labels: 5.2—Organic peroxide, Technical
Name Required. UN3108 : Organic peroxide type E, solid, Hazard
Class: 5.2; Labels: 5.2—Organic peroxide, Technical | [Incompatibilities]
May explode when heated above melting
point, 103 C. A strong oxidizer. Extremely explosionsensitive
to heat, shock, friction, and concussion. May
explode or cause fire on contact with reducing agents; combustible
substances, organic substances, wood, paper, metal
powders, lithium aluminum hydride. Violent reaction with
alcohols, organic and inorganic acids, and amines. | [Description]
Benzoyl Peroxide (94-36-0) may affect workers in the electronics and plastics (epoxy resins and catalysts) industries, electricians,
ceramic workers, dentists and dental technicians,
laboratory technicians and bakers. As it was
contained in candles, it also induced contact dermatitis
in a sacristan. However, some positive tests are of
unknown occupational relevance.
| [Waste Disposal]
Pretreatment involves decomposition
with sodium hydroxide. The final solution of
sodium benzoate, which is very biodegradable, may be
flushed into the drain. Disposal of large quantities of solution
may require pH adjustment before release into the
sewer or controlled incineration after mixing with a
noncombustible material. | [Definition]
ChEBI:Benzoyl peroxide is a carbonyl compound. | [Indications]
Benzoyl peroxide is a potent oxidizing agent that has
both antimicrobial and comedolytic properties; its primary
use is in treating acne vulgaris. It is converted in
the skin to benzoic acid; clearance of absorbed drug is
rapid, and no systemic toxicity has been observed. The
major toxicities are irritation and contact allergy.
Outgrowth of bacteria resistant to topical antibiotics
used to treat acne can be reduced by the addition of benzoyl
peroxide in combination products such as erythromycin
(Benzamycin) and clindamycin (Benzaclin). | [Brand name]
Acne-Aid Cream (Stiefel); Benoxyl (Stiefel); Benzac
(Galderma); Benzac W (Galderma); Brevoxyl (Stiefel);
Clear By Design (SmithKline Beecham); Dry and Clear
(Whitehall-Robins); Epi-Clear (Bristol-Myers Squibb);
Fostex BPO Bar, Gel, and Wash (Bristol-Myers Products);
Loroxide (Dermik); PanOxyl (Stiefel); Persa-Gel (Ortho
Pharmaceutical); Vanoxide (Dermik);Altex. | [World Health Organization (WHO)]
Benzoyl peroxide slowly releases oxygen and hence is
bactericidal. It is also keratolytic, antiseborrheic and irritant. It is used in the
treatment of acne. Benzoyl peroxide is listed in the WHO Model List of Essential
Drugs. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 28, p. 2168, 1963 DOI: 10.1021/jo01044a002 | [Health Hazard]
The health hazard from benzoyl peroxideis low. It can cause irritation of the skin,mucous membranes, and eyes. An intraperitonealinjection of 250 mg/kg was lethal toadult mice. Systemic toxicity in humans isnot known. It may be mild to moderatelytoxic on an acute basis. The oral LD50 valuein rats is 7710 mg/kg (NIOSH 1986). Itstoxicity from inhalation is low; an LC50 valueof 700 ppm in mice is suggested (ACGIH1986).
Benzoyl peroxide may cause gene damageand DNA inhibition. It has been foundto cause skin tumor. The evidence of its carcinogenicityin animals and humans is inadequate. | [Fire Hazard]
Benzoyl peroxide can cause a major fire
and explosion hazard. It is highly flammable
and a strong oxidizer; autoignition temperature
80°C (176°F). It ignites instantly. The
rate and violence of decomposition and the
potential ease of such ignition or decomposition
have been experimentally measured
by Noller et al. (1964). Lead pipe deformation
(LPD), pressure vessel test (PVT), and
self-accelerating decomposition test (SADT)
have been performed to measure these explosive
characteristics. Heating 5 g of benzoyl
peroxide in an aluminum tester containing an
aperture vent and 6-atm rupture disk, caused
the disk to blow up in 95 seconds when the
aperture vent area was less than 174.7 mm2.
Redried material was more violent. The
decomposition hazard was greatly reduced
with wet and diluted benzoyl peroxide.
Noller et al. (1964) measured the SADT
temperature at 82.2°C (180°F), above which
the decomposition was self-accelerating, sudden,
and produced smoke.
Benzoyl peroxide is a deflagrant, posing
a severe explosion hazard. The compound
is sensitive to heavy shock, such as impact
or blows, as well as to friction and heat.
Especially in the dry state, it is highly
dangerous.
A water sprinkler should be used to extinguish
fires. Water should be used to keep the
containers cool. | [Safety Profile]
Poison by intraperitoneal route.Can cause dermatitis, asthmatic effects, testicular atrophy,and vasodilation. An allergen and eye irritant. Humanmutation data reported. Questionable carcinogen withexperimental tumorigenic data. Moderate fire hazard by | [Veterinary Drugs and Treatments]
Benzoyl peroxide products are used topically either as gels or in shampoos. Shampoos are generally used for seborrheas, greasy skin (seborrhea
oleosa), or crusty pyodermas (such as seborrheic dermatitis/pyoderma commonly seen in Cocker Spaniels). Gels may be useful
for treating recurrent localized skin infections (e.g., chin acne), localized Demodex lesions, superficial and deep pyodermas (adjunctive
therapy), seborrhea oleosa, and Schnauzer comedo syndrome.
Benzoyl peroxide possesses antimicrobial (especially antibacterial), keratolytic and antiseborrheic actions. It also is It has some mild
antipruritic activity and wound healing effects, and is thought to increase follicular flushing. Benzoyl peroxide’s antimicrobial activity is
due to the oxidative benzoyl peroxy radicals formed that disrupt cell membranes. | [Carcinogenicity]
When repeatedly applied to the
skin of mice, BPO was not carcinogenic . However,
benzoyl peroxide is a tumor promoter in mice and hamsters,
but has shown no complete carcinogenic or tumor-initiating
activity . There has been one controversial Japanese
report that was interpreted as BPO being a complete
carcinogen. However, when the data were critically evaluated,
it was found consistent with BPO acting as a skin tumor
promoter and not as a carcinogen. The International Agency
for Research on Cancer (IARC) has evaluated the carcinogenicity
of benzoyl peroxide. They classified it as Group 3.
This means there is limited or inadequate evidence of carcinogenicity
for animals and inadequate or absent information
for humans. In addition, there are other animals and in vitro
studies that continue to support the lack of carcinogenic or
mutagenic properties for BPO . | [Source]
Benzoyl peroxide (BPO) was originally derived from chlorhydroxyquinoline, a component of coal tar. Currently, BPO is usually prepared by treating hydrogen peroxide with benzoyl chloride. | [storage]
Benzoyl peroxide should be stored in acool and well-ventilated area, isolated fromother chemicals and free of heating andelectrical installations. Dry compound maybe shipped in polyethylene-lined paper bagsor fiber containers packed in wooden boxeso. | [Purification Methods]
Dissolve benzoyl peroxide in CHCl3 at room temperature and precipitate it by adding an equal volume of MeOH or pet ether. Similarly it is precipitated from acetone by adding two volumes of distilled water. It has also been crystallised from 50% MeOH and from diethyl ether. Dry it under vacuum at room temperature for 24hours. Store it in a desiccator in the dark at 0o. When purifying in the absence of water it can be EXPLOSIVE, and operations should be done on a very small scale with adequate protection. Large amounts should be kept moist with water and stored in a refrigerator. [Kim et al. J Org Chem 52 3691 1987, Beilstein 9 IV 777.] |
| Safety Data | Back Directory | [Hazard Codes ]
O,Xn,N,Xi,E,T | [Risk Statements ]
R8:Contact with combustible material may cause fire.
R36/37/38:Irritating to eyes, respiratory system and skin .
R43:May cause sensitization by skin contact.
R36:Irritating to the eyes.
R2:Risk of explosion by shock, friction, fire or other sources of ignition.
R7:May cause fire.
R1:Explosive when dry.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R21/22:Harmful in contact with skin and if swallowed .
R62:Possible risk of impaired fertility.
R50:Very Toxic to aquatic organisms.
R61:May cause harm to the unborn child. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S17:Keep away from combustible material .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S60:This material and/or its container must be disposed of as hazardous waste .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S3/7:Keep container tightly closed in a cool place .
S14:Keep away from ... (incompatible materials to be indicated by the manufacturer) .
S47:Keep at temperature not exceeding ... E C (to be specified by the manufacturer) .
S35:This material and its container must be disposed of in a safe way .
S7:Keep container tightly closed .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [OEB]
B | [OEL]
TWA: 5 mg/m3 | [RIDADR ]
UN 3108 5.2
| [WGK Germany ]
2
| [RTECS ]
DM8575000 | [Autoignition Temperature]
176 °F | [TSCA ]
Yes | [HazardClass ]
5.2 | [PackingGroup ]
II | [HS Code ]
29163200 | [Hazardous Substances Data]
94-36-0(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 7710 mg/kg | [IDLA]
1,500 mg/m3 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Sodium hydroxide-->Hydrogen peroxide-->Benzoyl chloride-->Poly(acrylic acid)-->Benzoic acid-->Phenylacetone | [Preparation Products]
4-METHYL-2-OXO-2 H-CHROMENE-7-CARBALDEHYDE-->3-BROMO-2-((CYCLOPROPYLAMINO)METHYL)BENZALDEHYDE-->4-bromoisoindoline-->4-Bromomethylbenzamide-->ANION EXCHANGE RESIN 717-->3-CYANOMETHYLPHENYLBORONIC ACID-->(3-FLUORO-PHENYL)-METHANESULFONYL CHLORIDE-->(4-FLUORO-PHENYL)-METHANESULFONYL CHLORIDE-->7-BROMOMETHYL-4-METHYL-CHROMEN-2-ONE-->sulfonic ion exchange membrane based on polypropylene/poly (ST-DVB)-->5-BROMOISOINDOLINE-->POLY(VINYL ACETATE)-->Flour improver-->optical diskbase material modified PMMA copolymer-->Anagrelide-->Methyl 3-(bromomethyl)benzoate-->METHYL 2,5-DICHLOROTHIOPHENE-3-CARBOXYLATE-->4-(4-METHYLPIPERAZINOMETHYL)BENZOIC ACID-->alpha,alpha'-Dibromo-p-xylene-->METHYL ALPHA-BROMOPHENYLACETATE-->ETHOPERMETHRIN,95%-->the preparation and adsorption of poly (p-hydroxystyrene) adsorbents-->Perbenzoic acid-->Polymaleic acid-->Maleic acid-allyl alcohol copolymer-->Ozagrel-->4-Cyanobenzaldehyde-->2,5-DICHLOROTHIOPHENE-3-CARBONYL CHLORIDE-->3-Nitrobenzyl bromide-->2,5-DICHLOROTHIOPHENE-3-CARBOXYLIC ACID-->Ethyl 4-bromocrotonate-->1,4-BIS(TRICHLOROMETHYL)BENZENE-->N-CHLOROMETHYL-N-PHENYLCARBAMOYL CHLORIDE-->new Ni/carbonized resin catalyst-->POLYETHYLENE, CHLORINATED-->Methyl 2-bromomethylbenzoate-->2-(Bromomethyl)pyridine hydrobromide-->maleic anhydride-benzylethylene sulfonated acid copolyer-->ethyl 4,6,6,6-tetrachloro-3,3-dimethylhexanoate-->Naled |
| Questions And Answer | Back Directory | [Acne vulgaris]
Although the precise cause of acne is unclear, it appears to be associated with at least four factors: increased sebum production, follicular keratinisation, bacterial colonisation and inflammation.
Study suggests the prevalent bacterium implicated in the clinical course of acne is Propionibacterium acnes (P acnes), a gram-positive anaerobe that normally inhabits the skin and is implicated in the inflammatory phase of acne.
Benzoyl peroxide is mainly indicated in the treatment of mild to moderate acne and is often prescribed in conjunction with oral antibiotics (erythromycin or clindamycin) in the treatment of moderate to severe acne.
| [Active Ingredients for Acne Medications]
Benzoyl peroxide used in 2.5, 5, and 10 percent concentrations, depending on the acne severity. Usually these are in a gel spreading agent, but they can also be in a cream base or a drying paste. Benzoyl peroxide is a keratolytic, which means “keratin-dissolving” and works by loosening dead cells stuck in the follicles. It also releases oxygen in the follicle. Because acne bacteria are anaerobic, they cannot survive in the presence of oxygen. Benzoyl peroxide essentially works both as an interfollicular exfoliant and as an antibacterial.
| [Mode of action]
Benzoyl peroxide is lipophilic that can penetrate the stratum corneum and enter the pilosebaceous follicle. It is rapidly broken down to benzoic acid and hydrogen peroxide and generates free radicals that oxidise proteins in bacterial cell membranes, exerting a bactericidal action. In addition, it has shown that benzoyl peroxide can reduce the free fatty acid content of sebum, which provides a useful marker for bacterial activity. Benzoyl peroxide has an anti-inflammatory action and vitro studies suggest that this action arises from its ability to kill polymorphonuclear leukocytes (PMN cells) in the pilosebaceous follicles and so prevent their release of reactive oxygen species such as peroxides which enhance tissue inflammation. Involving equation about this process:
C6H5C(O)O-OC(O)C6H5 + H2O 2 C6H5COOH + ½ O2
Moreover, due to its irritant effect, benzoyl peroxide increases turnover rate of epithelial cells, thereby peeling the skin and promoting the resolution of comedones.
| [Side effects as Acne Treatment]
Skin reactions such as peeling, itching, irritation, and reddened skin may occur, especially at the start of treatment. A very serious allergic reaction to this drug is rare. This medicine may be harmful if swallowed.
| [Other Uses]
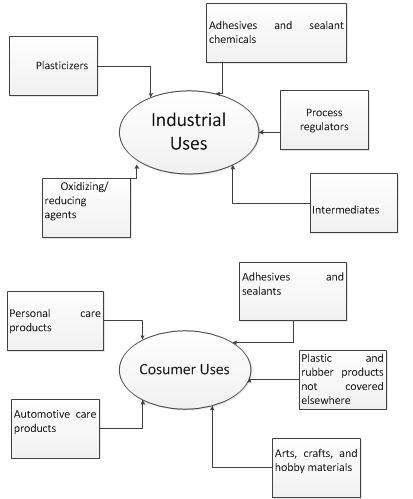
Benzoyl peroxide is used as a bleaching agent for certain foods, an oxidizing agent, a polymerizing initiator in the manufacture of plastics, a curing agent for silicone rubber, and an ingredient in various industrial processes.
Benzoyl peroxide, like most peroxides, is a powerful bleaching agent. It has a long history of use in the food industry as a bleaching agent added for flour, whey, and milk for cheese making. Contact with fabrics or hair can cause permanent color dampening almost immediately. Even secondary contact can cause bleaching.
Benzoyl peroxide is widely used as a catalyst in the polymerisation of molecules like styrene (phenylethene) to form polystyrene, which used to make many things from drinking cups to packaging material. | [Benzoyl peroxide and Pregnancy]
There are no studies looking at women who use topical benzoyl peroxide during pregnancy. When benzoyl peroxide is applied topically, only 5% is absorbed through the skin, and then it is completely metabolized to benzoic acid within the skin and excreted unchanged in the urine. It is not likely to increase risk for birth defects or cause problems for the baby. However, systemic effects on a pregnant woman and her child would not be expected and therefore use of this product during pregnancy would not be of concern.
| [References]
https://medlineplus.gov/druginfo/meds/a601026.html
https://pubchem.ncbi.nlm.nih.gov/compound/benzoyl_peroxide#section=Drug-and-Medication-Information
http://www.chm.bris.ac.uk/motm/benzoyl-peroxide/benzoylh.htm
https://www.webmd.com/drugs/2/drug-1344/benzoyl-peroxide-topical/details
https://pubchem.ncbi.nlm.nih.gov/compound/benzoyl_peroxide#section=Top
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3114665/
https://mothertobaby.org/fact-sheets/topical-acne-treatments-pregnancy/pdf/
|
|
|