| Identification | More | [Name]
2-Methoxynaphthalene | [CAS]
93-04-9 | [Synonyms]
2-METHOXYNAPHTHALENE
2-NAPHTHYL METHYL ETHER
BETA-METHYL NAPHTHYL ETHER
BETA NAPHTHOL METHYL ETHER
BETA-NAPHTHYL METHYL ETHER
B-NAPHTHOL METHYL ETHER
B-NAPHTHYL METHYL ETHER
METHYL 2-NAPHTHYL ETHER
METHYL B-NAPHTHYL ETHER
NAPROXEN IMP M
NEROLINE YARA YARA
NEROLIN YARA YARA
YARA YARA
2-methoxy-naphthalen
2-Naphthol methyl ether
2-naphtholmethylether
beta-Methoxynaphthalene
Ethyl beta-naphthyl ether
Jara jara
Methyl beta-naphthyl ether | [EINECS(EC#)]
202-213-6 | [Molecular Formula]
C11H10O | [MDL Number]
MFCD00004061 | [Molecular Weight]
158.2 | [MOL File]
93-04-9.mol |
| Chemical Properties | Back Directory | [Appearance]
white powder | [Melting point ]
70-73 °C (lit.) | [Boiling point ]
274 °C (lit.) | [density ]
1.064 g/mL at 25 °C(lit.)
| [vapor pressure ]
1.097Pa at 25℃ | [FEMA ]
4704 | BETA-NAPHTHYL METHYL ETHER | [refractive index ]
1.5440 (estimate) | [Fp ]
272-274°C | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
H2O: soluble (completely) | [form ]
Crystalline Solid | [pka]
0[at 20 ℃] | [color ]
White to yellow-brown | [Odor]
at 1.00 % in dipropylene glycol. sweet naphthyl floral orange blossom acacia neroli | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents. | [biological source]
synthetic | [Odor Type]
naphthyl | [Water Solubility ]
INSOLUBLE | [JECFA Number]
1257 | [Merck ]
14,5997 | [BRN ]
1859408 | [InChIKey]
LUZDYPLAQQGJEA-UHFFFAOYSA-N | [LogP]
3.318 at 25℃ | [CAS DataBase Reference]
93-04-9(CAS DataBase Reference) | [NIST Chemistry Reference]
Naphthalene, 2-methoxy-(93-04-9) | [EPA Substance Registry System]
93-04-9(EPA Substance) |
| Safety Data | Back Directory | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
UN 3077 9 / PGIII | [WGK Germany ]
2
| [RTECS ]
QJ9468750
| [TSCA ]
Yes | [HS Code ]
29093090 | [Toxicity]
LD50 oral in rat: > 5gm/kg |
| Hazard Information | Back Directory | [Description]
β-Naphthyl methyl ether has an intensely sweet, floral odor suggestive of orange blossoms. It is free from naphthol by-odor. It has a sweet, strawberry taste. This may be prepared from potassium β-naphthol and methyl chloride at 300°C; by methylation of β-naphthol with dimethyl sulfate or by direct esterification with methyl alcohol.
| [Chemical Properties]
Methyl 2-Naphthyl Ether forms white crystals (mp
73–74°C) with an intense orange blossom odor. | [Chemical Properties]
white powder | [Chemical Properties]
β-Naphthyl methyl ether has an intensely sweet, floral odor suggestive of orange blossoms; free from naphthol by-odor.
It has a sweet, strawberry taste | [Uses]
2-Methoxynaphthalene is an Impurity of the non-steroidal anti-inflammatory Naproxen (N377525). | [Definition]
ChEBI: 2-Methoxynaphthalene is a member of naphthalenes. | [Preparation]
From postassium β-naphthol and methyl chloride at 300°C; by methylation of β-naphthlol with dimethyl sulfate or by
direct esterification with methyl alcohol | [Synthesis Reference(s)]
Tetrahedron, 48, p. 6439, 1992 DOI: 10.1016/S0040-4020(01)88233-8
Tetrahedron Letters, 22, p. 3463, 1981 DOI: 10.1016/S0040-4039(01)81932-8 | [Flammability and Explosibility]
Notclassified | [Synthesis]
Preparation of 2-Methoxynaphthalene from 2-naphthol.
Principle: Phenols can be methylated to give methyl ethers. Methylation can be done either by using diazomethane or dimethyl sulphate in alkaline medium.
Reaction:
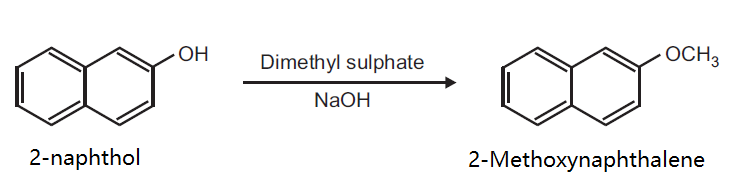
Procedure: Take 0.5 g 2-naphthol and 0.2 g NaOH in 5 ml distilled water in a beaker (25 ml). Heat on a wire gauze to obtain a clear solution. Cool the solution (10-15°C) and then add 0.35 ml dimethyl sulphate drop wise. After the addition is over, warm the mixture for one hour at 70-80°C and then cool. Filter the product and wash it with 10% sodium hydroxide solution and then with water. Dry the product, record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. Filter the white crystals of the product. Dry and record the melting point and TLC (using toluene as solvent).
| [Purification Methods]
Fractionally distil the ether under vacuum. Crystallise it from absolute EtOH, aqueous EtOH, *C6H6, pet ether or n-heptane, and dry it under vacuum in an Abderhalden pistol or distil it in vacuo. The picrate has m 118o (from EtOH or CHCl3). [Kikuchi et al. J Phys Chem 91 574 1987, Beilstein 6 III 2969, 6 IV 4257.] |
| Questions And Answer(Q&A) | Back Directory | [Chemical Synthesis]
Preparation of 2-Methoxynaphthalene from 2-naphthol.
Principle: Phenols can be methylated to give methyl ethers. Methylation can be done either by using diazomethane or dimethyl sulphate in alkaline medium.
Reaction:

Procedure: Take 0.5 g 2-naphthol and 0.2 g NaOH in 5 ml distilled water in a beaker (25 ml). Heat on a wire gauze to obtain a clear solution. Cool the solution (10-15°C) and then add 0.35 ml dimethyl sulphate drop wise. After the addition is over, warm the mixture for one hour at 70-80°C and then cool. Filter the product and wash it with 10% sodium hydroxide solution and then with water. Dry the product, record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. Filter the white crystals of the product. Dry and record the melting point and TLC (using toluene as solvent).
|
|
|