| Identification | Back Directory | [Name]
RDEA 594 | [CAS]
878672-00-5 | [Synonyms]
Lesinurd
RDEA 594
Recinard
Lesurinad
Lesinuras
Lesinurad
Lessinurad
Lacy Noulard
Lesinurad(RDEA5)
RDEA 594 USP/EP/BP
RDEA594(Lesinurad )
Lesinurad (RDEA-594)
Lesinurad impurity1-12
Lesinurad (with 3 ints.)
RDEA 594; RDEA-594; RDEA594
ethyl 3-(N-(5-chloro-2-nitrophenyl)anilino)-3-oxopropanoate
2-[[5-BroMo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl]thio]acetic acid
2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-1,2,4-triazol-3-yl]sulfanyl]acetic Acid
Acetic acid, 2-[[5-bromo-4-(4-cyclopropyl-1-naphthalenyl)-4H-1,2,4-triazol-3-yl]thio]-
2-{[5-broMo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl]sulfanyl}acetic acid
2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-1,2,4-triazol-3-yl]sulfanyl]acetic acid
Lesinurad | [EINECS(EC#)]
689-245-8 | [Molecular Formula]
C17H14BrN3O2S | [MDL Number]
MFCD22572730 | [MOL File]
878672-00-5.mol | [Molecular Weight]
404.281 |
| Chemical Properties | Back Directory | [Melting point ]
>171oC (dec.) | [Boiling point ]
643.7±65.0 °C(Predicted) | [density ]
1.72±0.1 g/cm3(Predicted) | [storage temp. ]
-20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Slightly, Heated) | [form ]
Solid | [pka]
2.91±0.10(Predicted) | [color ]
White to Off-White |
| Hazard Information | Back Directory | [Description]
Approved by the FDA late in
2015, lesinurad is an urate anion exchange transporter 1
(URAT1) inhibitor for use in the treatment of gout. Ardea
Biosciences, which is a subsidiary of AstraZeneca, developed
lesinurad to be used in a combination therapy with xanthine
oxidase inhibitors for the treatment of hyperuricaemia
associated with gout. The approval process is ongoing in
several other countries across the globe, with the EMA
Committee for Medicinal Products for Human Use giving
lesinurad a positive opinion for use as an adjunctive therapy in
combination with xanthine oxidase inhibitors to treat hyperuricaemia. | [Uses]
Lesinurad is used to synthesize febuxostat which is a non-purine analog inhibitor of xanthine oxidase. Febuxostat is approved by the European Medicines Agency and the US Food and Drug Administration for treating gout. | [Definition]
ChEBI: A member of the class of triazoles that is [(3-bromo-1,2,4-triazol-5-yl)sulfanyl]acetic acid substituted at position 1 of the triazole ring by a 4-cyclopropylnaphthalen-1-yl group. Used for treatment of gout. | [Synthesis]
The synthesis of lesinurad began with commercial 1-
bromonaphthalene (138). A Kumada coupling
between this bromide and cyclopropyl Grignard delivered 139,
which after selective nitration to give 140, delivered the oxylate
salt 141 (which now is commercially available). Treatment of
141 with KOH followed by thiophosgene at 5 ??C delivered
isothiocyanate 142 in 63% yield. Reaction of 142 with formyl
hydrazine followed by addition of potassium bicarbonate and
mild heating resulted in thio-1,2,4-triazole 144 by the
intermediacy of 143. Quantitative alkylation of triazolothiol
144 resulted in |á-mercaptan 145, and this was followed by
NBS bromination to afford bromotriazole 146. Ester saponification followed by acidification secured lesinurad
(XVII) in a good yield over the final three steps.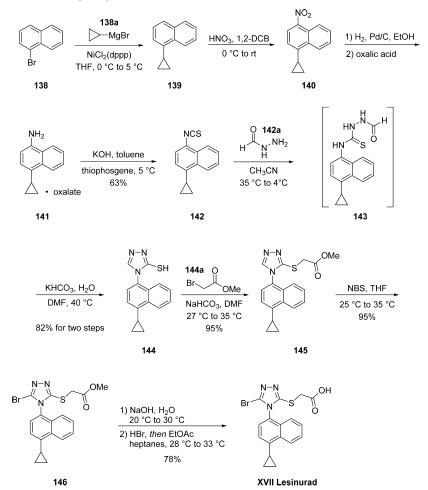
| [storage]
Store at -20°C |
|
|