| Identification | Back Directory | [Name]
CYCLIZINE HCL | [CAS]
82-92-8 | [Synonyms]
Valoid
bw47-83
Marzine
Emoquil
Marazine
Marezine
Bw 47-83
Wellcome
CYCLYZINE
Ciclizina
Nautazine
Ne-devomit
Neo-devomit
compound47-83
CYCLIZINE HCL
Cyclizine Base
Compound 47-83
Cyclizine USP/EP/BP
Wellcome prepn. 47-83
wellcomepreparation47-83
Wellcome preparation 47-83
n-benzhydryl-n-methylpiperazine
1-Benzhydryl-4-methylpiperazine
n-benzhydryl-n’-methylpiperazine
N-Benzhydryl-N-methyl piperazine
N-Benzhydryl-N'-methylpiperazine
N-Methyl-N'-benzhydrylpiperazine
n-methyl-n’-benzhydrylpiperazine
n-methyl-n’-benzyhydrylpiperazine
N-Methyl-N'-benzyhydrylpiperazine
1-Diphenylmethyl-4-methylpiperazine
1-(diphenylmethyl)-4-methyl-piperazin
Piperazine, 1-(diphenylmethyl)-4-methyl-
Cyclizine (base and/or unspecified salts)
(n-benzhydryl)(n’-methyl)diethylenediamine
(N-Benzhydryl)(N'-methyl)diethylenediamine
N-BENZHYDRYL-N'-METHYLPIPERAZINE HYDROCHLORIDE
1-DIPHENYLMETHYL-4-METHYL-PIPERAZINE HYDROCHLORIDE | [EINECS(EC#)]
201-445-5 | [Molecular Formula]
C18H23ClN2 | [MDL Number]
MFCD00067334 | [MOL File]
82-92-8.mol | [Molecular Weight]
302.84 |
| Chemical Properties | Back Directory | [Appearance]
White or almost white, crystalline powder. | [Melting point ]
105.5-107.5° | [Boiling point ]
399.58°C (rough estimate) | [density ]
0.9934 (rough estimate) | [refractive index ]
1.5840 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Slightly soluble in water and in ethanol (96 per cent). | [form ]
Solid | [color ]
White to off-white |
| Hazard Information | Back Directory | [Chemical Properties]
White or almost white, crystalline powder. | [Uses]
H1 antihistamine | [Definition]
ChEBI: An N-alkylpiperazine in which one nitrogen of the piperazine ring is substituted by a methyl group, while the other is substituted by a diphenylmethyl group. | [Brand name]
Marezine (GlaxoSmithKline). | [Originator]
Marezine,BurroughsWellcome,US,1953 | [Preparation]
Cyclizine is prepared through the alkylation of N-methylpiperazine with benzhydryl chloride. | [Manufacturing Process]
One-tenth mol (20 g) of benzhydryl chloride was mixed with 0.19 mol (19 g)
of N-methylpiperazine and about 10 cc of benzene and the whole was heated
on the steam bath four hours. The contents of the flask was partitioned
between ether and water, and the ethereal layer was washed with water until
the washings were neutral. The base was then extracted from the ethereal
layer by N hydrochloric acid and the extract, made acid to Congo red paper,
was evaporated under vacuum. 29.5 g of the pure dihydrochloride of N�methyl-N'-benzhydryl piperazine was recovered from the residue by
recrystallization from 95% alcohol melting above 250°C with decomposition.
The addition of alkali to an aqueous solution of the dihydrochloride liberated
the base which was recovered by recrystallization from petroleum ether
melting at 105.5° to 107.5°C. | [Therapeutic Function]
Antinauseant | [General Description]
Cyclizine hydrochloride,1-(diphenylmethyl)-4-methylpiperazine monohydrochloride(Marezine), occurs as a light-sensitive, white crystallinepowder with a bitter taste. It is slightly soluble in water(1:115), in alcohol (1:115), and in chloroform (1:75). It isused primarily in the prophylaxis and treatment of motionsickness. The lactate salt (Cyclizine Lactate Injection,United States Pharmacopoeia [USP]) is used for intramuscular(IM) injection because of the limited water solubilityof the hydrochloride. The injection should be stored in acold place, because if it is stored at room temperature forseveral months, a slight yellow tint may develop. This doesnot indicate a loss in biologic potency. | [Clinical Use]
It can be used not only in the treatment
of allergic diseases, but also in the management
of postoperative, irradiation or druginduced
vomiting. Cyclizine dependence has
been suggested to occur when it is used in combination
with analgesics in long-term therapy.
For this reason, several countries have removed
cyclicine from analgesic combination preparations. | [Synthesis]
Cyclizine, 1-(diphenylmethyl)-4-methylpiperazine (16.1.15), is synthesized by
alkylating 1-methylpiperazine with benzhydrylbromide.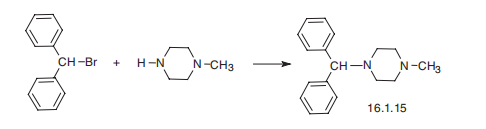
|
|
|