| Identification | More | [Name]
Methacrylic acid | [CAS]
79-41-4 | [Synonyms]
2-METHACRYLIC ACID
2-METHYLACRYLIC ACID
2-METHYLPROPENOIC ACID
MAA
METHACRYLIC ACID
PROPYLENE-2-CARBOXYLIC ACID
RARECHEM AL BO 0090
.alpha.-Methylacrylicacid
2-Methyl-2-propenoic acid
2-methyl-2-Propenoicacid
2-Methyl-2-propensαure
2-methyl-acrylicaci
2-methylene-propionicaci
2-methylenepropionicacid
2-Methylpropencicacid
2-Propenoicacid,2-methyl-
acidemethacrylique
acidemethacrylique(french)
acidometacrilico
Acrylic acid, 2-methyl- | [EINECS(EC#)]
201-204-4 | [Molecular Formula]
C4H6O2 | [MDL Number]
MFCD00002651 | [Molecular Weight]
86.09 | [MOL File]
79-41-4.mol |
| Chemical Properties | Back Directory | [Appearance]
colourless liquid or crystals with an unpleasant odour. | [Melting point ]
12-16 °C (lit.) | [Boiling point ]
163 °C (lit.) | [density ]
1.015 g/mL at 25 °C(lit.)
| [vapor density ]
>3 (vs air)
| [vapor pressure ]
1 mm Hg ( 20 °C)
| [refractive index ]
n20/D 1.431(lit.)
| [Fp ]
170 °F
| [storage temp. ]
Indoors | [solubility ]
Chloroform, Methanol (Slightly) | [form ]
Liquid | [pka]
pK1:4.66 (25°C) | [color ]
Clear | [Odor]
Repulsive | [PH]
2.0-2.2 (100g/l, H2O, 20℃) | [Stability:]
May be stabilized by the addition of MEHQ (Hydroquinone methyl ether, ca. 250 ppm) or hydroquinone. In the absence of a stabilizer this material will readily polymerize. Combustible. Incompatible with strong oxidizing agents, hydrochloric acid. | [explosive limit]
1.6-8.7%(V) | [Water Solubility ]
9.7 g/100 mL (20 ºC) | [Sensitive ]
Moisture & Light Sensitive | [Merck ]
14,5941 | [BRN ]
1719937 | [Exposure limits]
TLV-TWA 20 ppm (~70 mg/m3) (ACGIH). | [InChIKey]
CERQOIWHTDAKMF-UHFFFAOYSA-N | [LogP]
0.93 at 22℃ | [Uses]
Monomer for large-volume resins and polymers, organic synthesis. Many of the polymers are
based on esters of the acid, as the methyl, butyl, or
isobutyl esters. | [CAS DataBase Reference]
79-41-4(CAS DataBase Reference) | [NIST Chemistry Reference]
2-Propenoic acid, 2-methyl-(79-41-4) | [EPA Substance Registry System]
79-41-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R21/22:Harmful in contact with skin and if swallowed .
R35:Causes severe burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [OEB]
A | [OEL]
TWA: 20 ppm (70 mg/m3) [skin] | [RIDADR ]
UN 2531 8/PG 2
| [WGK Germany ]
1
| [RTECS ]
OZ2975000
| [Autoignition Temperature]
752 °F | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29161300 | [Safety Profile]
Poison by
intraperitoneal route. Moderately toxic by
ingestion and skin contact. Corrosive to
skin, eyes, and mucous membranes.
Mutation data reported. Flammable when
exposed to heat, flame, or oxidizers. A
storage hazard; exothermic polymerization
may occur spontaneously. To fight fire, use
alcohol foam, spray, mist, dry chemical.
When heated to decomposition it emits
acrid smoke and irritating fumes. | [Hazardous Substances Data]
79-41-4(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 1320 mg/kg |
| Hazard Information | Back Directory | [General Description]
A clear colorless liquid (or low-melting solid) with a pungent odor. Corrosive to metals and tissue. Flash point 170°F. Melting point 61°F. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently. Less dense than water. Vapors heavier than air. Used to make plastics. | [Reactivity Profile]
METHACRYLIC ACID(79-41-4) reacts with strong oxidizing agents. Presents a storage hazard: violent exothermic polymerizations leading to explosion can occur spontaneously, particularly at low inhibitor or stabilizer concentrations [Anon., CISHC Chem. Safety Summ., 1979, 50, p. 34; Bond, J., Loss Prev. Bull., 1991, 101, p. 1]. | [Air & Water Reactions]
Soluble in water. | [Hazard]
Toxic material. Strong irritant to eyes and
skin. Questionable carcinogen. | [Health Hazard]
INHALATION: Severe irritation to respiratory tract. EYES: Short contact can cause severe damage. SKIN: Causes severe irritation and burns. Ingestion: High hazard-may cause death or permanent injury on short exposure to small quantities. OTHER: May affect blood pressure temporarily. | [Potential Exposure]
Methacrylic acid is used in preparation of methacrylates and carboxylated polymers; in the
production of the material or its alkyl esters, as monomers
or comonomers for synthetic resins for the production of
plastic sheets, moldings, and fibers. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water or
milk. Do not induce vomiting. Medical observation is
recommended for 24 to 48 hours after breathing overexposure, as pulmonary edema may be delayed. As first aid for
pulmonary edema, a doctor or authorized paramedic may
consider administering a drug or other inhalation therapy. | [Shipping]
UN2531 Methacrylic acid, stabilized, Hazard
class: 8; Labels: 8-Corrosive material. | [Incompatibilities]
Vapor may form explosive mixture
with air. A reducing agent; incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or
explosions. Aqueous solution is strongly acidic: incompatible
with strong acids; caustics, ammonia, amines, isocyanates,
alkylene oxides; epichlorohydrin. Will polymerize readily from
heating above 59�F/15�C, or due to the presence of light, oxidizers (e.g., peroxides); or in the presence of traces of hydrochloric acid, with fire or explosion hazard. Attacks metals. Note:
Typically contains 100 ppm of monomethyl ether hydroquinone (150-76-5) as an inhibitor to prevent polymerization | [Description]
Methacrylic acid, abbreviated MAA, is an organic compound. This colourless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA) and poly(methyl methacrylate) (PMMA). The methacrylates have numerous uses, most notably in the manufacture of polymers with trade names such as Lucite and Plexiglas. MAA occurs naturally in small amounts in the oil of Roman chamomile. | [Chemical Properties]
colourless liquid or crystals with an unpleasant odour. | [Chemical Properties]
Methacrylic acid is a colorless liquid. | [Waste Disposal]
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. | [Definition]
ChEBI: An alpha,beta-unsaturated monocarboxylic acid that is acrylic acid in which the hydrogen at position 2 is substituted by a methyl group. | [Preparation]
The most common route for the preparation of methacrylic acid is from
acetone as follows:
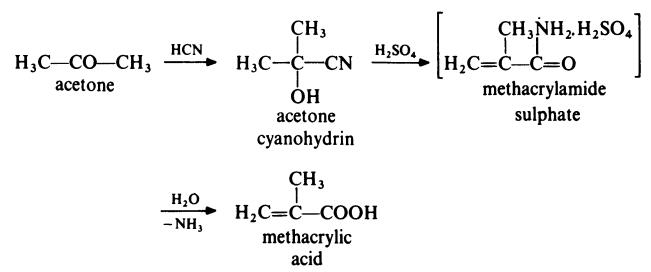
In a typical process, acetone is treated with hydrogen cyanide at 140??C in the
presence of ammonia as catalyst. The acetone cyanohydrin produced is
treated with concentrated sulphuric acid at 100??C to form methacrylamide
sulphate. This intermediate is not isolated but is directly converted to
methacrylic acid by treatment with water at about 90??C.
A competitive route now in commercial operation involves the two stage
oxidation of isobutene with air. The reaction proceeds via methacrolein:
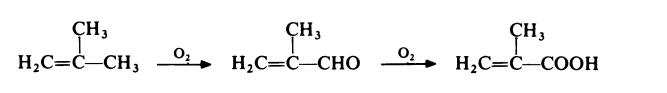
| [Production Methods]
More than 3 million tons of methyl methacrylate (MMA) are produced annually. In one route, acetone cyanohydrin is converted to methacrylamide sulfate using sulfuric acid. That compound is hydrolyzed to methacrylic acid, or it can be converted into methyl methacrylate in one step. In the second route, isobutylene or tertbutanol are oxidized to methacrolein, then methacrylic acid.Methacrolein for this purpose can also be obtained from formaldehyde and ethylene. Isobutyric acid can also be dehydrogenated to methacrylic acid.
Methacrylic acid was first obtained in the form of its ethyl ester by treating phosphorus pentachloride with oxyisobutyric ester.[2] It is, however, more readily obtained by boiling citra- or mesobrompyrotartaric acids with alkalis. It crystallizes in prisms. When fused with an alkali, it forms propanoic acid. Sodium amalgam reduces it to isobutyric acid. A polymeric form of methacrylic acid was described in 1880. | [Fire Hazard]
Combustible liquid; flash point (open cup)
76°C (170°F); vapor pressure <0.1 torr at
20°C (68°F). Fire-extinguishing agent: water
spray, “alcohol” foam, dry chemical, or CO2;
use a water spray to dilute and flush the spill
and to disperse the vapors.
Methacrylic acid polymerizes readily. The
reaction is exothermic. The rate of reaction
accelerates on heating, which may result
in violent rupture of closed containers. The
polymerization may be inhibited with a trace
quantity of hydroquinone and hydroquinone
monomethyl ether (Aldrich 2006). The acid
may be stored safely below its melting point. | [Carcinogenicity]
Methacrylic acid was considered
by the IARC Working Groups, but monographs were not
prepared because adequate data on its carcinogenicity were
not available. The IUCLID database reports a
dermal application study (dose unspecified) of mice treated
three times per week for 4 months and then observed for their
lifetimes. No excess dermal tumors were observed. | [storage]
(1) Color Code—White: Corrosive or Contact Hazard; Store separately in a corrosion-resistant location. (2) Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. (3) Color Code—Yellow Stripe (strong reducing agent): Reactivity Hazard; Store separately in a area isolated from flammables, combustibles, or other yellow coded materials. Prior to working with this chemical you should be trained on its proper handling and storage. Before entering confined space where this chemical may be present, check to make sure that an explosive concentration does not exist. Store in tightly closed containers in a cool, well-ventilated area away from oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates). Methacrylic acid should be stored at temperatures below 15℃. Sources of ignition, such as smoking and open flames are prohibited where methacrylic acid is handled, used, or stored. Wherever methacrylic acid is used, handled, manufactured, or stored, use explosion-proof electrical equipment and fittings. | [Purification Methods]
Aqueous methacrylic acid (90%) is saturated with NaCl (to remove the bulk of the water), then the organic phase is dried with CaCl2 and distilled under vacuum. Polymerisation inhibitors should be added to the distillate and include 0.25% p-methoxyphenol, 0.1% hydroquinone, or 0.05% N,N'-diphenyl-p-phenylenediamine. [Beilstein 2 IV 1518.] | [Toxics Screening Level]
The current ITSL for methacrylic acid (30 μg/m3) has a justification (attached) dated September 13, 2007. |
|
|