| Identification | More | [Name]
Tetraethyl orthosilicate | [CAS]
78-10-4 | [Synonyms]
AKOS BBS-00004380
ETHYL ORTHOSILICATE
ETHYL-O-SILICATE
ETHYL SILICATE
ORTHOSILICIC ACID TETRAETHYL ESTER
SILICON TETRAETHOXIDE
TEOS
TETRAETHOXYSILANE
TETRAETHYL ORTHOSILICATE
TETRAETHYL SILICATE
(C2H5O)4Si
Dynasil A
dynasila
ES 28 Ester
es100
es28
es28(ester)
Ethyl silicat
Ethyl silicate, ((EtO)4Si)
Etylu krzemian | [EINECS(EC#)]
201-083-8 | [Molecular Formula]
C8H20O4Si | [MDL Number]
MFCD00009062 | [Molecular Weight]
208.33 | [MOL File]
78-10-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Ethyl silicate is a colorless, flammable liquid
with a sharp odor detectable @ 85 ppm | [Melting point ]
-77 °C
| [Boiling point ]
168 °C(lit.)
| [density ]
0.94 | [vapor density ]
7.2 (vs air)
| [vapor pressure ]
<1 mm Hg ( 20 °C)
| [refractive index ]
n20/D 1.382(lit.)
| [Fp ]
116 °F
| [storage temp. ]
Flammables area | [solubility ]
Soluble in ethanol and 2-propanol. | [form ]
Liquid | [color ]
Colorless | [Specific Gravity]
0.934 | [Stability:]
Stable. Flammable. Incompatible with strong oxidizing agents, water, alkalies, mineral acids. | [explosive limit]
1.3-23%(V) | [Water Solubility ]
Hydrolysis | [FreezingPoint ]
-77℃ | [Hydrolytic Sensitivity]
7: reacts slowly with moisture/water | [Sensitive ]
Moisture Sensitive | [Detection Methods]
GC,NMR | [Merck ]
14,3851 | [BRN ]
1422225 | [Dielectric constant]
4.1(20℃) | [Exposure limits]
ACGIH: TWA 10 ppm
OSHA: TWA 100 ppm(850 mg/m3)
NIOSH: IDLH 700 ppm; TWA 10 ppm(85 mg/m3) | [InChIKey]
BOTDANWDWHJENH-UHFFFAOYSA-N | [CAS DataBase Reference]
78-10-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Silicic acid (H4sio4), tetraethyl ester(78-10-4) | [Storage Precautions]
Moisture sensitive | [EPA Substance Registry System]
78-10-4(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
Ethyl silicate is a flammable, colorless liquid with a mild, sweet, alcohol-like odor. Exposure to ethyl silicate can occur through inhalation, ingestion, and eye or skin contact. It is practically insoluble in water, soluble in alcohol, and slightly soluble in benzene. Occupational workers are exposed to ethyl silicate at workplaces associated with the manufacture and transportation of ethyl silicate, during use as a bonding agent for industrial buildings and investment castings, ceramic shells, crucibles, refractory bricks, and other molded objects, as a protective coating for heatand chemical-resistant paints, lacquers, and fi lms, in the manufacture of protective and preservative coatings for protection from corrosion (primarily as a binder for zinc dust paints), chemicals, heat, scratches, and fi re. Workers are also exposed to the chemical substance in the production of silicones; as a chemical intermediate in the preparation of soluble silica; as a gelling agent in organic liquids, as a coating agent inside electric lamp bulbs, in the synthesis of fused quartz, and during industrial use in the textile industry in aqueous emulsions, deluster, and fi reproofi ng; as a component of lubricants; as a mold-release agent; and as a heat-resistant adhesive. | [General Description]
A clear colorless liquid with a faint odor. Flash point 125°F. Less dense than water. Vapors are heavier than air. | [Reactivity Profile]
ETHYL SILICATE(78-10-4) reacts exothermically with acids Strong oxidizing acids may cause a reaction that is sufficiently exothermic to ignite the reaction products. May generate with caustic solutions. May generate flammable hydrogen with alkali metals and hydrides. | [Air & Water Reactions]
Flammable. Practically insoluble in water. Reacts slowly with water to form silica and ethyl alcohol [Merck]. | [Hazard]
Moderate fire risk. Strong irritant to eyes,
nose, throat. | [Health Hazard]
Exposures to ethyl silicate cause adverse health effects. The symptoms of poisoning include, but are not limited to, irritation of the eye, mucous membrane, respiratory tract, respiratory diffi culty, tremor, fatigue, narcosis, nausea, and vomiting. Prolonged periods of skin contact may produce drying, cracking, infl ammation, and dermatitis. As observed in laboratory animals, occupational workers exposed to the chemical substance may suffer from liver and kidney damage, CNS depression, and anemia. At concentrations of 3000 ppm, ethyl silicate causes extreme and intolerable irritation of the eyes and mucous membranes; at 1200 ppm, it produces tearing of the eyes; at 700 ppm, it causes mild stinging of the eyes and nose; and at 250 ppm, it produces slight irritation of the eyes and nose.
| [Health Hazard]
Inhalation of vapor causes eye and nose irritation, unsteadiness, tremors, salivation, respiratory difficulty, and unconsciousness. Contact with liquid irritates eyes and may cause dryness, cracking, and inflammation of skin. Ingestion may produce nausea, vomiting, and cramps. | [Potential Exposure]
Ethyl silicate is used as a binder in
production of cases and molds for investment casting of
metals. The next largest application is in corrosion-resistant
coatings; primarily as a binder for zinc dust paints.
Miscellaneous uses include the protection of white-light
bulbs; the preparation of soluble silicas; catalyst preparation and regeneration; and as a crosslinker and intermediate
in the production of silicones | [Fire Hazard]
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Medical observation is recommended for 24 to 48 hours
after breathing overexposure, as pulmonary edema may be
delayed. As first aid for pulmonary edema, a doctor or
authorized paramedic may consider administering a drug or
other inhalation therapy. | [Shipping]
UN1292 Tetraethyl acetate, Hazard Class: 3;
Labels: 3-Flammable liquid. | [Incompatibilities]
May form explosive mixture with air.
Strong oxidizers; strong acids; water. | [Waste Disposal]
Incineration in admixture
with a more flammable solvent. | [Production Methods]
Prepared from absolute alcohol and silicon tetrachloride. | [Purification Methods]
Fractionate it through an 80cm Podbielniak type column (p 11) with a heated jacket and partial take-off head. It is slowly decomposed by H2O-and is soluble in EtOH. It is flammable-it irritates the eyes and mucous membranes. [Sumrell & Ham J Am Chem Soc 78 5573 1956, Bradley et al. J Chem Soc 5020 1952, Beilstein 1 IV 1360.] |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R10:Flammable.
R20:Harmful by inhalation.
R36/37:Irritating to eyes and respiratory system . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
UN 1292 3/PG 3
| [WGK Germany ]
1
| [RTECS ]
VV9450000
| [Autoignition Temperature]
230 °C | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29209090 | [Precautions]
Occupational workers should avoid contact between ethyl silicate and strong oxidizers, water, mineral acids, and alkalis. Workers should use appropriate personal protective clothing and equipment that must be carefully selected, used, and maintained to be effective in preventing skin contact with ethyl silicate. The selection of the appropriate personal protective equipment (PPE) (e.g., gloves, sleeves, encapsulating suits) should be based on the extent of the worker’s potential exposure to ethyl silicate. There are no published reports on the resistance of various materials to permeation by ethyl silicate. | [Safety Profile]
Poison by intravenous
route. Moderately toxic by other routes. A
skin,mucous membrane, and severe eye
irritant. Narcotic in high concentrations.
Flammable liquid when exposed to heat or
flame; can react vigorously with oxidzing
materials. When heated to decomposition it
emits acrid smoke and fumes. See also
ESTERS. | [Hazardous Substances Data]
78-10-4(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 6270 mg/kg LD50 dermal Rabbit 5860 mg/kg | [IDLA]
700 ppm |
| Questions And Answer | Back Directory | [Ethyl silicate]
Ethyl silicate is also known as Tetraethyl orthosilicate,colorless, transparent liquid with special smell. Stable under the condition of anhydrous, when encountering water, it decomposes into ethanol and silicic acid, cloudy in moist air, soluble in alcohol, ether and other organic solvents. It is toxic, strong irritative to the human eye and respiratory tract. it is prepared by distillation after the reaction of silicon tetrachloride with ethanol. It is used for producing heat and chemical resistant coatings and preparing silicone solvent, can also be used in organic synthesis, the basic raw material for preparing advanced crystal, used as optical glass processing agent, binders, insulation materials for electronics industry, etc.
Ethyl silicate itself is not able to bind, if ethyl silicate is used as refractories binding agents, it must be hydrolyzed before use. TEOS hydrolysis reactions under conditions of water only is very slow, if that is under catalytic action of acid (H +) or base (OH-) catalysis, hydrolysis rate is greatly accelerated. Hydrochloric acid is generally used as a catalyst, as if alkali is as a catalyst, hydrolytic gel will happen soon in hydrolysis solution, leaving the hydrolytic sol destabilized, and thus lose the ability to bind, ethyl silicate hydrolysis under acid catalysis is as follows:
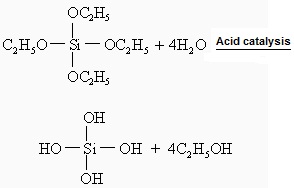
The hydrolysis is essentially the ethoxy (C2H5O-)of ethyl silicate is substituent by hydroxyl (-OH) of water, with the result that ethyl silicate (Si4-OC2H5) converted into a silanol group (Si4-OH). Silanol are highly active, can continue to conduct acid exchange reaction or etherification reaction with other silicic acid ethyl or silanols.
However, the extent of the hydrolysis reaction is carried out by a certain control, to form a stable hydrolyzate of ethyl silicate. Otherwise, the results of continuous reaction will form a body polyorganosiloxane and lose stability, it becomes insoluble gel, thus lose workability. Stability of ethyl silicate hydrolyzate is adjusted mainly by adding acid or base. When the pH is between 1.5 and 2.5, the gel occurs for a longer time, hydrolyzate is most stable. Lower or higher than this range, hydrolysis prone to gel, the pH is 5-6, the hydrolyzate prone to gel and is most unstable. Thus, the general hydrolyzate should be controlled between 2.0 and 2.5, in order to maintain its stability to maintain a certain working time (the time of construction or molding) after mixed with refractory material. Ethyl silicate hydrolyzate can be used as die casting refractory binding agents, also binding agents for clay, high alumina, corundum, containing zircon, mullite, silicon carbide and castable products.
The above information is edited by the chemicalbook of Yan Yanyong. | [Chemical Properties]
Ethyl silicate is a flammable, colourless liquid with a mild, sweet, alcohol-like odour. Exposure to ethyl silicate can occur through inhalation, ingestion, and eye or skin contact. It is practically insoluble in water, soluble in alcohol, and slightly soluble in benzene.
| [Uses]
1. Tetraethyl orthosilicate is Used as insulating materials in electronic industry, also used for optical glass processing and used as coagulants.
2. For precision casting, as a sand binder. Metal surface treated by ethyl silicate vapor can be anticorrosion and waterproof. Ethyl silicate can be used to bleed on the metal surface of the silicon, processing optical glass can improve its light transmittance. Fine silica powder produced by complete hydrolysis can be used to manufacture the phosphor. Ethyl silicate is raw material for silicone oil. Ethyl silicate can also be used to manufacture heat-resistant, chemical-resistant coatings. In Japan, 90% of ethyl silicate is used as an anti-corrosion coating (zinc-rich paint) base material.
3. Tetraethyl orthosilicate is Mainly used in chemical-resistant coatings and heat-resistant coatings, used as silicone solvent and precision-made binder. Fine silica powder produced after complete hydrolysis, used for the manufacture of phosphor, also used as a chemical reagent.
4. Tetraethoxysilane is mainly used in optical glass, chemical resistant coatings and heat-resistant coatings and adhesives, modification for anti-corrosion coating, used as crosslinking agent, a binder, a dehydrating agent, used for manufacturing catalyst skeleton, high purity ultrafine silica.
5. Used as Insulating materials in electronic industry, coatings, optical glass treatment agents, coagulants, used for organic synthesis, used as solvents for the preparation of organosilicon. | [Production method]
It is produced by esterification of silicon tetrachloride with ethanol at normal temperature and pressure.
| [Category]
Flammable liquids.
| [Toxicity grading]
Poisoning
| [Acute toxicity]
Oral-rat LD50: 6270 mg/kg, Inhalation-rat LCL0: 85 g/cubic meter.
| [Stimulus data]
Skin-rabbit 500 mg/24 hours moderate. Eyes-rabbit 500 mg/24 hours mild.
| [Storage Characteristics]
Treasury ventilation low-temperature drying, stored separately from oxidants.
| [Extinguishing agent]
Foam, powder, carbon dioxide, sand.
| [Occupational standards]
TWA 85 mg/m3, STEL 170 mg/m3.
|
|
|