| Identification | More | [Name]
Enalapril | [CAS]
75847-73-3 | [Synonyms]
1-[2-(1-ETHOXYCARBONYL-3-PHENYL-PROPYL)AMINOPROPANOYL]PYRROLIDINE-2-CARBOXYLIC ACID
1-(n-((s)-1-carboxy-3-phenylpropyl)-l-alanyl)-l-proline 1'-ethyl ester
ENALAPRIL
(s)-1-(n-(1-(ethoxycarbonyl)-3-phenylpropyl)-l-alanyl)-l-proline
Renitec
Vasotec
Enalapril Maleate USP24
L-Proline, 1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-, (S)-
L-Proline, N-[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl-
(S)-1-[N-[1-(Ethoxyearbonyl)-3-phenylpropyl]-L-alanyl]-L-proline
G1ioten
Hipoartd
(2S)-1-[(2S)-2-[[(2S)-1-Ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]pyrrolidine-2-carboxylic acid
(S)-N-[1-Ethoxycarbonyl)-3-phenylpropyl]-Ala-Pro
(-)-1-[N-[(S)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-L-proline
1-[N-[(S)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-L-proline
N-[(1S)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-Ala-L-Pro-OH
N-[(S)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-Ala-L-Pro-OH | [Molecular Formula]
C20H28N2O5 | [MDL Number]
MFCD00865774 | [Molecular Weight]
376.45 | [MOL File]
75847-73-3.mol |
| Hazard Information | Back Directory | [Uses]
angiotensin converting enzyme inhibitor | [Uses]
Enalapril is used in the treatment of hypertension, diabetic nephropathy, and some types of chronic heart failure. It has been proven to protect the function of the kidneys in hypertension, heart failure, and diabetes, and may be used in the absence of hypertension for its kidney protective effects. It is widely used in chronic kidney failure. | [Uses]
It is used for hypertension and chronic cardiac insufficiency. | [Definition]
ChEBI: A dicarboxylic acid monoester that is ethyl 4-phenylbutanoate in which a hydrogen alpha to the carboxy group is substituted by the amino group of L-alanyl-L-proline (S-configuration). | [Mechanism of action]
Like captopril, enalapril selectively suppresses the rennin–angiotensin–aldosterone system,
inhibits angiotensin-converting enzyme, and prevents conversion of angiotensin I into
angiotensin II. | [Synthesis]
Enalapril, (S)-1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-L-proline
(22.7.12), is synthesized by reacting the benzyl ester of L-alanyl-L-proline with the ethyl
ester of 3-benzoylacrylic acid, which forms the product 22.7.11, the reduction of which
with hydrogen using a Raney nickel catalyst removes the protective benzyl group, giving
the desired enalapril (22.7.12) [24]. Alternative methods of syntheses have also been proposed.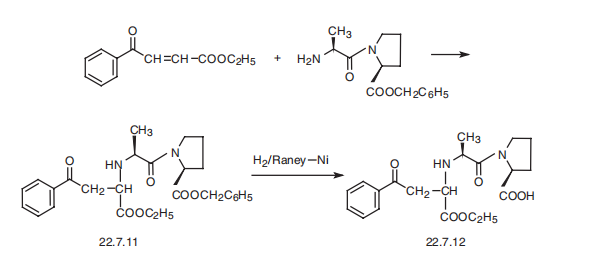
| [storage]
Store at -20°C |
|
|