| Identification | Back Directory | [Name]
2-(2H-Benzotriazol-2-yl)-6-(1-methyl-1-phenylethyl)-4-(1,1,3,3-tetramethylbutyl)phenol | [CAS]
73936-91-1 | [Synonyms]
UV-928
TINUVIN 928
LOTSORB UV 928
Absorber UV-928
UV ABSORBER-928
UV ABSORBER TRUELICHT UV 928
2-6(2H-Benzotriazol-2-yl)-6-(1,1,3,3-tetramethylbutyl)Phenol
2-[2-Hydroxy-3-dimethylbenzylphenyl-5-(1,1,3,3-tetramethylbutyl)]-2H-benzotriazol
2-[2-HYDROXY-3-DIMETHYLBENZYLPHENYL-5-(1,1,3,3-TETRAMETHYLBUTYL)]-2H-BENZOTRIAZOLE
2-(benzotriazol-2-yl)-6-(2-phenylpropan-2-yl)-4-(2,4,4-trimethylpentan-2-yl)phenol
2-(2H-BENZOTRIAZOL-2-YL)-6-(1-METHYL-1-PHENYLETHYL)-4-(1,1,3,3-TETRAMETHYLBUTYL)PHENOL
2-(2H-benzotriazol-2-yl)-6-(1-methyl-1-phenylethy)-4-(1,1,3,3-tetramethylbutyl)-Phenol
Phenol, 2-(2H-benzotriazol-2-yl)-6-(1-methyl-1-phenylethyl)-4-(1,1,3,3-tetramethylbutyl)-
2-(2H-Benzo[d][1,2,3]triazol-2-yl)-6-(2-phenylpropan-2-yl)-4-(2,4,4-trimethylpentan-2-yl)phenol
2-(2H-Benzotriazol-2-yl)-6-(1-methyl-1-phenylethyl)-4-(1,1,3,3-tetramethylbutyl)phenol ISO 9001:2015 REACH | [EINECS(EC#)]
231-545-4 | [Molecular Formula]
C29H35N3O | [MDL Number]
MFCD02100741 | [MOL File]
73936-91-1.mol | [Molecular Weight]
441.608 |
| Chemical Properties | Back Directory | [Melting point ]
112 °C | [Boiling point ]
555.5±60.0 °C(Predicted) | [density ]
1.07 | [solubility ]
soluble in Toluene | [form ]
powder to crystal | [pka]
8.05±0.50(Predicted) | [color ]
White to Orange to Green | [InChI]
InChI=1S/C29H35N3O/c1-27(2,3)19-28(4,5)21-17-22(29(6,7)20-13-9-8-10-14-20)26(33)25(18-21)32-30-23-15-11-12-16-24(23)31-32/h8-18,33H,19H2,1-7H3 | [InChIKey]
UZUNCLSDTUBVCN-UHFFFAOYSA-N | [SMILES]
C1(O)=C(C(C)(C2=CC=CC=C2)C)C=C(C(C)(C)CC(C)(C)C)C=C1N1N=C2C=CC=CC2=N1 | [EPA Substance Registry System]
Phenol, 2-(2H-benzotriazol-2-yl)-6-( 1-methyl-1-phenylethyl)-4-( 1,1,3,3-tetramethylbutyl)- (73936-91-1) |
| Questions And Answer | Back Directory | [Description]
UV absorber-928(C29H35N3O) is a hydroxyphenyl benzotriazole UV absorber specially designed for high performance coating applications. UV absorber-928 provides efficiency to coating and light sensitive substrates due to its characteristic broad band absorption. With excellent solubility and high thermal and environmental performance, UV absorber-928 is particularly suitable for coatings exposed to high temperature curing processes, such as powder and coil coatings, or high environmental stress.
| [Uses]
UV absorber-928 has broad absorption properties which can effectively protect coating and other photosensitive materials. UV absorber-928 has high solubility, high temperature resistant and good environmental durability and so on. UV absorber-928 is suitable for high performance coatings, especially suitable for powder coating and industrial and automotive coatings. UV absorber-928 can be enhanced when used in combination with a HALS stabilizer such as UV-292 or UV-123.
|
| Hazard Information | Back Directory | [Chemical Properties]
light yellow crystalline powder | [Application]
UV-928 has broad absorption properties which can effectively protect coating and other photosensitive materials.The product has high solubility, high temperature resistant and good environmental durability and so on..It is suitable for high performance coatings, especially suitable for powder coating and industrial and automotive coatings. UV-928 can be enhanced when used in combination with a HALS stabilizer such as UV-292 or UV-123. | [Flammability and Explosibility]
Notclassified | [Synthesis]
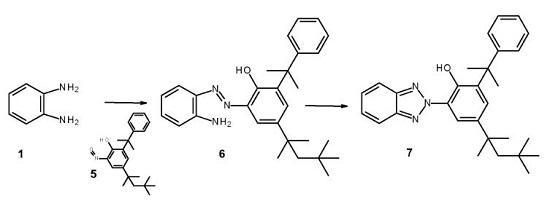
In a dry argon atmosphere 0.150 g of oxime 5, 0.070 g of 1,2-phenylene diamine 1 and 0.150
ml triethyl-borate are dissolved in 4 ml dry THF and heated for 24 h at 60°C. After a TLC
shows consumption of starting oxime the mixture is evaporated to dryness and the resulting
residue purified by column chromatography (eluent: hexane - ethyl acetate: 10-1 vol/vol) to
give 0.171 g (92 %) of the diazo-amine intermediate 6.
Replacing the B(OEt)3 by 2.5 g of basic AI2O3 and the THF by 2 ml of xylene and heating this
mixture to 140°C for 48 h gives after the usual work-up 0.089 mg of diazo compound 6 and
0.300 g of starting oxime 5.
According the procedure given for the preparation of compound 4 in example 1, triazole 7 is
obtained from 0.007 g (0.016 mmol) of intermediate amine 6 and 0.020 g (0.122 mmol) CuSO4
in 100% (0.007 g). In this comparative example, 7.73 equivalents of copper salt are used. |
|
|