| Identification | More | [Name]
(R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone | [CAS]
72332-33-3 | [Synonyms]
PROCATEROL
(r*,s*)-(-)-8-hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1h)-quinolinone
2(1h)-quinolinone,8-hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-,(r*,
8-hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1h)-quinolinone(r*,s
s*)-(+-)- | [EINECS(EC#)]
276-590-0 | [Molecular Formula]
C16H22N2O3 | [MDL Number]
MFCD01750015 | [Molecular Weight]
290.36 | [MOL File]
72332-33-3.mol |
| Hazard Information | Back Directory | [Description]
Like pirbuterol, procaterol exhibits similar broncholytic properties as albuteral, but it has
somewhat of a more prolonged action. It is recommended for use as an inhaled drug for
treating bronchial asthma. | [Chemical Properties]
Off-White Solid | [Uses]
A labelled intermediate of the enantiomer of Florfenicol (F405750), an antibacterial agent. | [Definition]
ChEBI: 8-hydroxy-5-[1-hydroxy-2-(propan-2-ylamino)butyl]-1H-quinolin-2-one is a member of quinolines. | [Brand name]
Pro-Air (Parke-Davis). | [Synthesis]
Procaterol, 5-[1-hydroxy-2-[(1-methylethyl)amino]butyl]-8-hydroxy-2-(1H)
quinolone (23.3.25), is synthesized by acylation of 8-hydroxy-2(1H)-quinolone with 2-bromobutyric acid chloride at the fifth position of the quinoline system, which gives the
compound 23.3.23. This undergoes action of isopropylamine, forming an aminoketone
23.3.24, the carbonyl group of which is reduced by sodium borohydride, giving procaterol
(23.3.25) .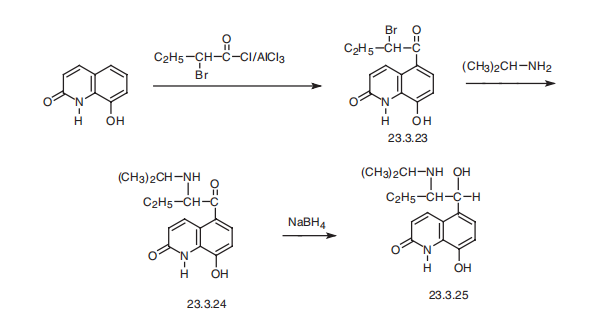
|
|
| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Website: |
https://www.bocsci.com |
| Company Name: |
BOC Sciences
|
| Tel: |
16314854226 |
| Website: |
www.bocsci.com |
|