| Identification | More | [Name]
Naftifine hydrochloride | [CAS]
65473-14-5 | [Synonyms]
(e)-n-cinnamyl-n-methyl(1-naphthylmethyl)amine hydrochloride
NAFTIFENE HYDROCHLORIDE
NAFTIFINE HCL
NAFTIFINE HYDROCHLORIDE
N-TRANS-CINNAMYL-N-METHYL-(1-NAPHTHYLMETHYL)AMINE HYDROCHLORIDE
Exoderil,Naftin(R),Suadian
Naftifine hyrdrochloride
Exoderil, Naftin, Suadian,
NAFTINE HCL | [EINECS(EC#)]
620-502-9 | [Molecular Formula]
C21H22ClN | [MDL Number]
MFCD00059047 | [Molecular Weight]
323.86 | [MOL File]
65473-14-5.mol |
| Chemical Properties | Back Directory | [Appearance]
White Powder | [Melting point ]
172-175°C | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [solubility ]
ethanol: soluble50mg/mL | [form ]
Solid | [color ]
White to Off-White | [Usage]
An antifungal agent | [Merck ]
14,6355 | [InChI]
InChI=1S/C21H21N.ClH/c1-22(16-8-11-18-9-3-2-4-10-18)17-20-14-7-13-19-12-5-6-15-21(19)20;/h2-15H,16-17H2,1H3;1H/b11-8+; | [InChIKey]
OLUNPKFOFGZHRT-YGCVIUNWSA-N | [SMILES]
N(C/C=C/C1=CC=CC=C1)(C)CC1=C2C(C=CC=C2)=CC=C1.[H]Cl | [CAS DataBase Reference]
65473-14-5(CAS DataBase Reference) |
| Questions And Answer | Back Directory | [Local antifungal agents]
Naphthalene hydrochloride, also known as cinnamonyl methylamine, naphthalene, and naphthalene hydrochloride, is currently one of the commonly used drugs for fungal infection in Department of Dermatology, belonging to the local antifungal agents of acrylamines. It has bactericidal effect on the dermatophytes (Trichophyton, Microsporum and epidermis Trichophyton). It has bacteriostasis against Malassezia, Candida and other yeasts. It also has local bactericidal effect on Gram-positive and negative bacteria. The mechanism of its action is to inhibit the fungal squalene cyclooxygenase. It interferes with the biosynthesis of ergosterol in fungal cell wall. It affects the lipid metabolism of fungi and causes the damage or death of fungal cells to play a bactericidal and bacteriostasis effect. It is suitable for dermatophytosis caused by sensitive fungi, such as tinea corporis, tinea pedis, tinea capitis, onychomycosis, tinea versicolor, superficial candidiasis as well as erasing fungal disease in the skin folds. For the the external application of 1% Naftifine Hydrochloride Ointment on the skin by thehealthy people, about 3% to 6% of the doses can be absorbed.The concentration of naphthofinn on the surface of the skin at a single dose within 24 hours is sufficient to inhibit the growth of dermatitis. Naftifine can be converted to at least three metabolites through the oxidation of benzene ring and naphthalene and N- alkylation in the body. About 40% to 60% of the drugs in the body are excreted in the form of the original and metabolite form into the urine, and the rest is excreted into the feces by the bile. The half-life of the skin external use of naftifine is about 2~3 days.
Usage and dosage: external use. For the skin infection, usually 1 times /d, and gently rub. The skin within 2.5cm centred on the infection should be coated with the drug. For erasing fungal disease, a ribbon gauze containing this product can be disposed at night in wrinkles.
Duration of treatment: 2-4 weeks for dermatophyte infection and 8 weeks in severe cases; 4 weeks for superficial moniliasis;6 months for Onychomycosis ; 2 weeks for pityriasis versicolor. For the prevention of recurrence, the treatment continues for about 2 weeks after all the signs of infection have disappeared.
| [Uses]
Used as antibiotic drugs, antifungal agents with broad spectrum and strong effect. The curative effect is good and fast with high the safety and survivability. It is for the treatment of dermatophyte infection, superficial moniliasis, Onychomycosis and skin and its accessories (hair finger, fingernail and toe) by achorion, microspore and epidermis Trichophyton. Indications include erasing mycosis of the lower breast as well as erasing mycosis between finger (toes), the buttocks, and the groin.This product has the characteristics of broad antifungal spectrum and low toxicity.
|
| Hazard Information | Back Directory | [Description]
Naftifine hydrochloride(65473-14-5) is a topical antifungal agent which acts via inhibiting
squalene epoxidase. It demonstrates good activity against Trichophyton,
Epidermophyton, Microsporum, Aspergillus, and species. It is the
prototype of a significantly improved series of antifungal agents, exemplified by
SF 86-327 in which the phenyl group of naftifine has been replaced by
t-butylace tylene.
| [Chemical Properties]
White Powder | [Originator]
Sandoz (Switzerland) | [Definition]
ChEBI: Naftifine hydrochloride is a hydrochloride and an allylamine antifungal drug. | [Brand name]
Naftin (Merz);EXODERIL. | [General Description]
N-Methyl-N-(3-phenyl2-propenyl)-1-naphthalenemethanaminehydrochloride (Naftin) is a white crystallinepowder that is soluble in polar solvents such asethanol and methylene chloride. It is supplied in a 1% concentrationin a cream and in a gel for the topical treatmentof ringworm, athlete’s foot, and jock itch. Although unapprovedfor these uses, naftifine has shown efficacy fortreatment of ringworm of the beard, ringworm of the scalp,and tinea versicolor. | [Clinical Use]
Naftifine hydrochloride (Naftin,65473-14-5) is available for topical
use only in the treatment of cutaneous dermatophyte
and Candida infections; it is as effective as topical
azoles for these conditions.
| [Synthesis]
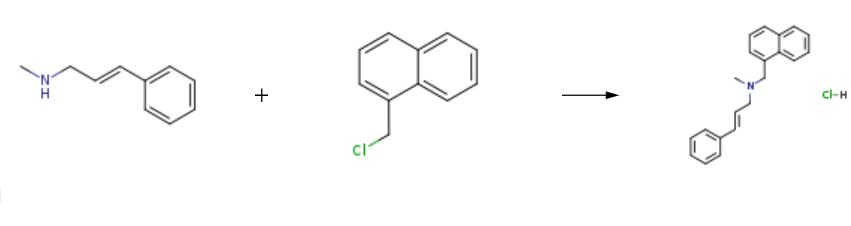
Naftifine hydrochloride is prepared by the reaction of (E)-N-methylcinnamylamine and 1-Chloromethylnaphthalene. The steps are as follows:
Step 1: (E)-N-methylcinnamylamine; 1-Chloromethylnaphthalene With sodium hydroxide In toluene at 86℃; for 6h;
Step 2: With hydrogenchloride In water; toluene at 15 - 20℃; for 3.5h�;
Step 3: Add toluene (75 mL) to the yellow oil of step 2 (trans-N-cinnamomethylamine crude)And 15% NaOH (9.8g),Warming up,Stir well,When the temperature rises to 86 ° C,1-Chloromethylnaphthalene (11.8 g,100.2mmol), dripping,The reaction was incubated at 86 ° C for 6 h.Medium controlled N-cinnamylmethylamine is less than 0.5%,Cool down to 30 ° C, add water (75mL) to the system,Stirring and standing, separating the organic phase,The organic phase was washed with water (75 mL).Allow to stand and separate the organic phase, Concentrated hydrochloric acid (7.0 g) was added to the organic phase in a 15 ° C ice water bath.Adjust the pH to about 2 and stir for 0.5 h.After stirring at 20 ° C for 3 h, the filter cake was filtered.The filter cake was rinsed with toluene (20 mL).Obtained a wet cake (reduced naftifine hydrochloride) 19.5g;The wet crude product obtained in step 3 (crude naftifine hydrochloride) was added to isopropyl alcohol (38.6 g).Warmed to 85 ° C, dissolved,Slowly cool after dissolving,The cooling rate is 10 °C / h,A small amount of solid precipitated at 35 ° C.Crystallization at this temperature for 2 h,Continue to cool down to 20 ° C,Insulation for 3h,filter,Get the wet cake,The filter cake was dried under vacuum at 45 ° C.Obtained 16.2g of finished naftifine hydrochloride,The purity of the finished naftifine hydrochloride was 99.4% (HPLC normalization method).The product yield was 68.0%.The product yield is the molar yield,The calculation is as follows:Product yield (%)={m/[(m0/M0)*M]}*100%Symbol Description: m is the mass of the finished product, g; m0 is the mass of cinnamyl alcohol, g; M is the molar mass of naftifine hydrochloride,g/mol; M0 is the molar mass of cinnamyl alcohol, g/mol.
 |
|
|