| Identification | More | [Name]
Pipracil | [CAS]
61477-96-1 | [Synonyms]
PIPERACILLIN NA
PIPERACILLIN SODIUM
PIPERACILLIN SODIUM SALT
3-dioxo-1-piperazinyl)carbonyl)amino)phenylacetyl)amino)-7-oxo-l-(2s-(2-al
4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylicacid,3,3-dimethyl-6-(((((4-ethy
6-(d-(-)-alpha-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)phenylacetamido)pe
[2S-[2alpha,5alpha,6beta(S*)]]-6-[[[[(4-ethyl-2,3-dioxopiperazin-1-yl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
PIPERACILLIN EPP(CRM STANDARD)
PIPERACILLIN USP(CRM STANDARD)
PIPERAILLIN
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)-(9CI)
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-, [2S-[2a,5a,6b(S*)]]-
Piperacillin (base and/or unspecified salts)
(2S,5R,6R)-6-[[(2R)-[[(4-Ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
Piperacillin
Pipracil
6α-[[(R)-(4-Ethyl-2,3-dioxo-1-piperazinylcarbonylamino)phenylacetyl]amino]penicillanic acid
6α-[[(R)-α-Oxo-β-[[(2,3-dioxo-4-ethyl-1-piperazinyl)carbonyl]amino]phenethyl]amino]penicillanic acid
PIPC
Pipercillin | [EINECS(EC#)]
262-811-8 | [Molecular Formula]
C23H27N5O7S | [MDL Number]
MFCD00917471 | [Molecular Weight]
517.55 | [MOL File]
61477-96-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
183-185?C (dec.) | [density ]
1.51±0.1 g/cm3(Predicted) | [vapor pressure ]
0Pa at 20℃ | [RTECS ]
XH8952200 | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
Freely soluble in methanol. Only sparingly soluble in aqueous solution at 0.119 mg/mL | [form ]
Solid | [pka]
2.44±0.50(Predicted) | [color ]
White to Off-White | [Water Solubility ]
256.8mg/L at 20℃ | [Stability:]
Hygroscopic | [LogP]
-1.55 at 20℃ | [CAS DataBase Reference]
61477-96-1(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Crystalline Solid | [Originator]
Pentcillin, Toyama ,Japan ,1980 | [Uses]
antibactierial | [Uses]
Broad spectrum semi-synthetic antibiotic related to Penicillin. Antibacterial. | [Definition]
ChEBI: A penicillin in which the substituent at position 6 of the penam ring is a 2-[(4-ethyl-2,3-dioxopiperazin-1-yl)carboxamido]-2-phenylacetamido group. | [Manufacturing Process]
To a suspension of 0.9 g of 6-[D(-)-α-aminophenylacetamido]penicillanic acid in 30 ml of anhydrous ethyl acetate were added at 5°C to 10°C 0.55 g of triethylamine and 0.6 g of trimethylsilyl chloride. The resulting mixture was reacted at 15°C to 20°C for 3 hours to form trimethylsilylated 6-[D(-)-αaminophenylacetamido]penicillanic acid.
To this acid was then added 1 g of 4-ethyl-2,3-dioxo-1-piperazinocarbonyl chloride (from the reaction of N-ethylethylenediamine and diethyl oxalate to give 2,3-dioxo-4-ethyl-piperazine which is then reacted with phosgene) and the resulting mixture was reacted at 15°C to 20°C for 2 hours. After the reaction, a deposited triethylamine hydrochloride was separated by filtration, and the filtrate was incorporated with 0.4 g of n-butanol to deposit crystals. The deposited crystals were collected by filtration to obtain l.25 g of white crystals of 6-[D(-)α-(4-ethyl-2,3-dioxo-1-piperazinocarbonylamino) phenylacetamido]penicillanic acid. Into a solution of these crystals in 30 ml of tetrahydrofuran was dropped a solution of 0.38 g of a sodium salt of 2-ethylhexanoic acid in 10 ml of tetrahydrofuran, upon which white crystals were deposited. The deposited crystals were collected by filtration, sufficiently washed with tetrahydrofuran and then dried to obtain 1.25 g of sodium salt of 6-[D(-)-α-(4-ethyl-2,3-dioxo-1-piperazinocarbonylamino)phenylacetamido] penicillanic acid, melting point 183°C to 185°C (decomposition), yield 90%. | [Brand name]
Pipracil (Wyeth). | [Therapeutic Function]
Antibiotic | [Antimicrobial activity]
It displays good activity against non-β-lactamaseproducing
strains of N. gonorrhoeae, ampicillin-susceptible
H. influenzae and many Enterobacteriaceae. It is the most
active of the antipseudomonal penicillins against Ps. aeruginosa
and retains its activity in the absence of a β-lactamase
inhibitor. Synergy with aminoglycosides has been demonstrated
against many strains of Enterobacteriaceae and Ps.
aeruginosa. | [Acquired resistance]
There is complete cross-resistance with other ureidopenicillins,
but ticarcillin-resistant strains of Ps. aeruginosa may
be susceptible. Piperacillin-resistant strains of B. fragilis
and other Bacteroides spp. are common. Because piperacillin
is hydrolyzed by most β-lactamases, many β-lactamaseproducing
isolates are resistant unless it is protected by
β-lactamase inhibitors. | [Flammability and Explosibility]
Notclassified | [Pharmacokinetics]
Oral absorption: Negligible
Cmax 2 g (2–3 min intravenous injection): 305 mg/L after 5 min
Plasma half-life: 0.9 h
Volume of distribution: 16–24 L/1.73 m2
Plasma protein binding: 16%
In patients with meningitis, mean CSF penetration of 30%
has been found. The urine is the principal route of excretion,
50–70% of the dose appearing over 12 h, most in the first
4 h. Most is excreted via the tubules, 75–90% in active form.
The half-life is prolonged in renal failure but much less than
is the case with carboxypenicillins. There is substantial biliary
excretion, levels in the common duct bile after a 1 g intravenous
dose commonly reaching 500 mg/L or more. During
hemodialysis the plasma half-life remains elevated and only
10–15% of the dose is removed. | [Clinical Use]
Intra-abdominal infection
Urinary tract infections
Gynecological and gonococcal infections
Septicemia
Lower respiratory infections
Skin and skin structure infections
Bone and joint infections | [Clinical Use]
Piperacillin (Pipracil) is the most generally useful of the extended-spectrum acylureidopenicillins. It is more active thanmezlocillin against susceptible strains of Gram-negativeaerobic bacilli, such as Serratia marcescens, Proteus,Enterobacter, Citrobacter spp., and P. aeruginosa.Mezlocillin, however, appears to be more active againstProvidencia spp. and K. pneumoniae. Piperacillin is alsoactive against anaerobic bacteria, especially B. fragilis andS. faecalis (enterococcus). β-Lactamase–producing strainsof these organisms are, however, resistant to piperacillin,which is hydrolyzed by S. aureus β-lactamase. The β-lactamase susceptibility of piperacillin is not absolute becauseβ-lactamase–producing, ampicillin-resistant strainsof N. gonorrhoeae and H. influenzae are susceptible topiperacillin.
Piperacillin is destroyed rapidly by stomach acid; therefore,it is active only by intramuscular or intravenousadministration. The injectable form is provided as the white,crystalline, water-soluble sodium salt. Its pharmacokineticproperties are very similar to those of the other acylureidopenicillins. | [Side effects]
Piperacillin is generally well tolerated, with mild to moderate
pain on injection, thrombophlebitis and diarrhea in some
patients. It otherwise exhibits side effects common to the
group, including hypersensitivity, leukopenia and abnormalities
of platelet aggregation without coagulation defect, except
on prolonged treatment. | [Synthesis]
Piperacillin, (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2R)-2-[(4-ethyl-2,3-dioxo-
1-piperazinyl)formamido]-2-phenylacetamido]-4-thia-1-azabicyclo[3.2.0]-heptan-2-car�boxylic acid (32.1.1.30), is also synthesized by acylating ampicillin (32.1.1.16), but with
1-chlorocarbonyl-4-ethylpiperazin-2,3-dione (32.1.1.29). The necessary 1-chlorocarbonyl-4-
ethylpiperazin-2,3-dione (32.1.1.29) is synthesized by reacting N-ethylethylenediamine with
diethyloxalate, forming 4-ethylpiperazin-2,3-dione (32.1.1.28), and then acylating this with
phosgene after initial silylation of the product at the nitrogen atom with trimethylchlorosilane.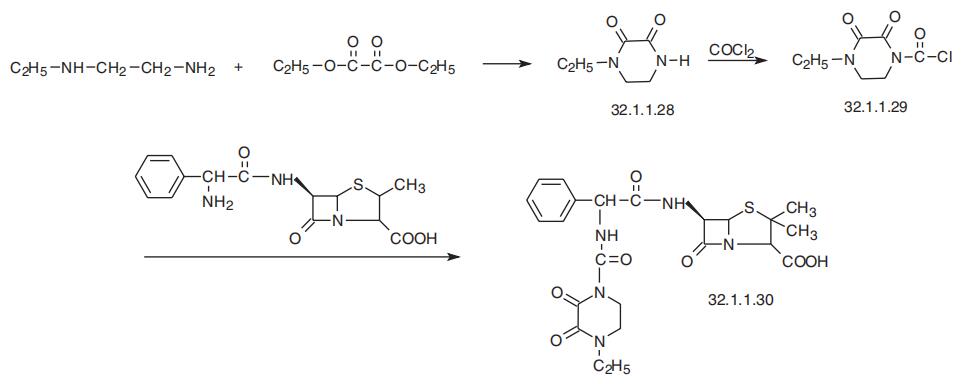
| [Mode of action]
Piperacillin binds to penicillin binding proteins (PBP) located on the inner membrane of the bacterial cell wall, thereby interfering with the cross-linking of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. As a result, cell wall synthesis is interrupted leading to a weakened cell wall and eventually cell lysis. |
|
|