| Identification | More | [Name]
CYCLOHEXANE | [CAS]
608-80-0 | [Synonyms]
CYCLOHEXANE
CYCLOHEXANE-195
CYCLOHEXANE-205
HEXAHYDROBENZENE
HEXAHYDROXYBENZENE
HEXAMETHYLENE
HEXANAPHTHALENE
HEXANAPHTHENE
NAPHTHENE
Benzene-1,2,3,4,5,6-hexol | [EINECS(EC#)]
203-806-2 | [Molecular Formula]
C6H12 | [MDL Number]
MFCD00003814 | [Molecular Weight]
84.16 | [MOL File]
608-80-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
4-7 °C(lit.)
| [Boiling point ]
80.7 °C(lit.)
| [density ]
0.779 g/mL at 25 °C(lit.)
| [vapor density ]
2.9 (vs air)
| [vapor pressure ]
168.8 mm Hg ( 37.7 °C)
| [refractive index ]
n20/D 1.426(lit.)
| [Fp ]
-1 °F
| [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [form ]
powder to crystal | [pka]
9.17±0.33(Predicted) | [color ]
White to Gray to Brown | [InChIKey]
VWPUAXALDFFXJW-UHFFFAOYSA-N | [CAS DataBase Reference]
608-80-0(CAS DataBase Reference) | [EPA Substance Registry System]
Benzenehexol (608-80-0) |
| Safety Data | Back Directory | [Hazard Codes ]
F,Xn,N | [Risk Statements ]
R11:Highly Flammable.
R38:Irritating to the skin.
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R65:Harmful: May cause lung damage if swallowed.
R67:Vapors may cause drowsiness and dizziness. | [Safety Statements ]
S9:Keep container in a well-ventilated place .
S16:Keep away from sources of ignition-No smoking .
S25:Avoid contact with eyes .
S33:Take precautionary measures against static discharges .
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S62:If swallowed, do not induce vomiting: seek medical advice immediately and show this container or label . | [RIDADR ]
UN 1145 3/PG 2
| [WGK Germany ]
2
| [RTECS ]
GU6300000
| [F ]
3-10 | [HS Code ]
2907.29.0500 |
| Hazard Information | Back Directory | [Synthesis]
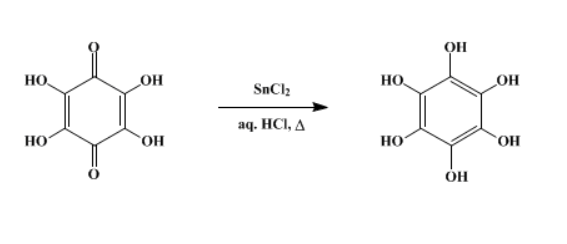
Hexahydroxy-benzene is synthesised using tetrahydroxyquinone as raw material by chemical reaction. The specific synthesis steps are as follows:
Step 1: One hundred grams (0.44 mole) of stannous chloride dihydrate is added to a boiling solution of 10 g. (0.058 mole) of tetrahydroxyquinone in 200 ml. of 2.4N hydrochloric acid contained in a 1.5-l. beaker. The initial deep-red color disappears, and grayish crystals of hexahydroxybenzene precipitate. Two hundred fifty milliliters of 12N hydrochloric acid is added, and the mixture is heated to boiling with constant stirring. The beaker is removed from the hot plate, an additional 600 ml. of 12N hydrochloric acid is added, and the solution is cooled in a refrigerator. The hexahydroxybenzene is collected on a Büchner funnel fitted with a sintered-glass disk and sucked dry.
Step 2: The crude hexahydroxybenzene is dissolved in 450 ml. of hot 2.4N hydrochloric acid containing 3 g. of hydrated stannous chloride and 1 g. of decolorizing carbon. The solution is filtered while hot, and the carbon is rinsed with 75 ml. of boiling water that is combined with the filtrate. One liter of 12N hydrochloric acid is added, and the mixture is cooled in a refrigerator. The snow-white crystals of hexahydroxybenzene that separate are collected under carbon dioxide or nitrogen on a Büchner funnel fitted with a sintered-glass disk. The hexahydroxybenzene is washed with 100 ml. of a cold 1:1 mixture of ethanol and 12N hydrochloric acid and dried in a vacuum desiccator over sodium hydroxide pellets; yield 7.1–7.8 g. (70–77%). It fails to melt on a hot plate at 310°.
 |
|
|